Unauthorized skin lightening and skin treatment products may pose serious health risks
Summary
Stop using these products and consult your health care professional if you have health concerns. Report these or any unauthorized health products to Health Canada.
Affected products
| Photo | Product & (Promoted Use) | Hazard Identified | Company | Action Taken | Date Added |
|---|---|---|---|---|---|
 |
Activ Clobe Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
BETACET-N Cream (Skin treatment) | Labelled to contain betamethasone dipropionate and neomycin | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Brilliant Skin Essentials Rejuvenating Facial Cream | Product tested by Health Canada and found to contain tretinoin and hydroquinone | Camrose Luxe Shoppe Camrose, AB |
Removed from the warehouse location and from online sale | 2023-04-19 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set | Labelled to contain hydroquinone and tretinoin | Dorton's Online shop.yyc Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
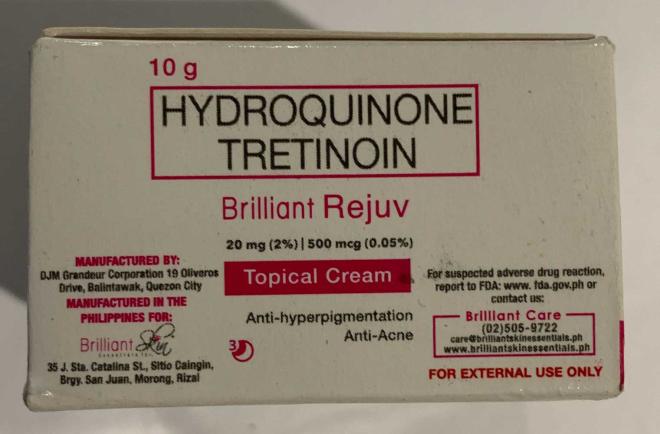
Brilliant Skin Essentials Brilliant Rejuv Topical Cream | Labelled to contain hydroquinone and tretinoin | Dorton's Online shop.yyc Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
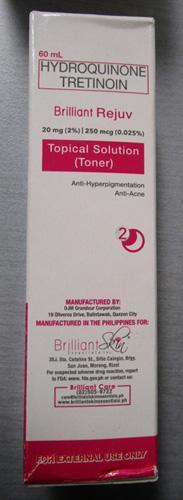
Brilliant Skin Essentials Brilliant Rejuv Topical Solution (Toner) |
Labelled to contain hydroquinone and tretinoin | Dorton's Online shop.yyc Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream | Labelled to contain hydroquinone and tretinoin | Camrose Luxe Shoppe Camrose, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Solution (Toner) | Labelled to contain hydroquinone and tretinoin | Camrose Luxe Shoppe Camrose, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
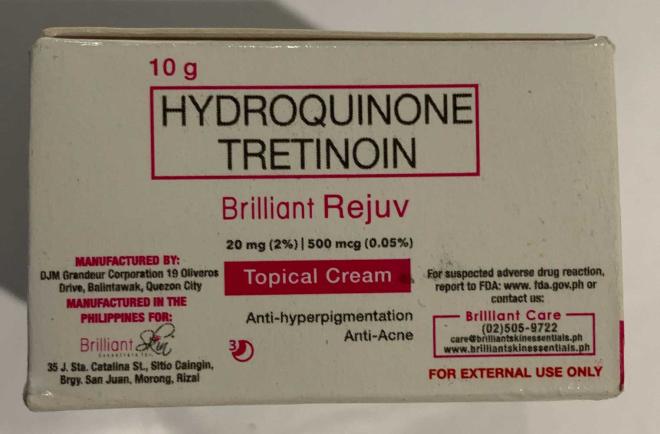
Brilliant Skin Essentials Brilliant Rejuv Topical Cream | Labelled to contain hydroquinone and tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
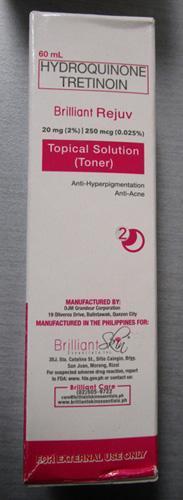 |
Brilliant Skin Essentials Brilliant Rejuv Topical Solution (Toner) | Labelled to contain hydroquinone and tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set | Labelled to contain hydroquinone and tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
 |
Brilliant Skin Essentials Rejuvenating Facial Toner | Product with similar packaging (previously removed from sale) was tested and found to contain tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
 |
Dermaglow Extra Whitening Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Dermaglow Extra Whitening Face Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Dermo Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Globatin antiMarks Complexion Lightening Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Medvate Cream (Skin whitening) | Labelled to contain betamethasone valerate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Betnovate-N 25 g (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Desi Mandi Store 1515 N Service Rd Burlington, ON |
Seized from retail location | 2023-05-18 |
 |
Betnovate-N 20 g (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Desi Mandi Store 1515 N Service Rd Burlington, ON |
Seized from retail location | 2023-05-18 |
 |
LS BL Cream (Skin treatment) | Labelled to contain ketoconazole and clobetasol propionate | Bags Clothing and Beyond Rosthern, SK |
Removed from sale | 2023-05-18 |
Issue
Health Canada is advising about unauthorized health products for lightening skin or treating skin conditions (such as eczema or psoriasis) skin lightening products that may pose serious health risks. The products are labelled to contain or have been tested and found to contain dangerous ingredients. Health Canada updates the table when it finds unauthorized health products for skin lightening. Links to previous tables with affected products are also available below.
Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality. Unauthorized health products can pose many health dangers, including:
- They may contain ingredients not listed on the label. This includes ingredients like prescription drugs, possibly at doses exceeding maximum recommended amounts. Prescription drugs should be taken only under the supervision of a health professional because they may cause serious side effects. Using a product that contains ingredients that the consumer is not aware of increases the chance of dangerous allergies and interactions with other medications and foods.
- The label may indicate a dangerous ingredient or combination of ingredients. For example, it could list a drug that should be available only by prescription from a health care professional, or a combination of ingredients that Health Canada does not permit because of serious health risks.
Health Canada maintains this page so that the public can easily identify products they may have purchased and take appropriate action. You are encouraged to check back regularly for updates. Advisories on safety issues involving other types of products are available in the recalls and safety alerts database.
What you should do
- Stop using the products listed in the table. Consult your health care professional if you have used these products and have health concerns, and for advice on which health products are best for you and your family.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check if products have been authorized for sale by searching Health Canada’s Drug Product Database and Licensed Natural Health Product Database.
- Report any health product adverse events or complaints to Health Canada.
- See these helpful links for additional information on buying health products safely:
What companies should know:
- Selling unauthorized health products in Canada is illegal.
- When Health Canada identifies unauthorized products that may pose serious health risks, Health Canada takes appropriate action to prevent further distribution and informs the public. This includes working with the Canada Border Services Agency to help prevent further importation of unauthorized products.
Additional information
Previous recalls or alerts
Background
Hydroquinone is a prescription drug when it is greater than a 2% concentration and a natural health product at a concentration of 2% and under. It is used topically (applied to the skin) to lighten areas of darkened skin caused by different conditions (e.g., sun exposure, skin damage, pregnancy, medications or age). It should not be used by people who are allergic to hydroquinone or who are taking medicines that make their skin more sensitive to light. Hydroquinone is not recommended for pregnant or breastfeeding people, or for children. It should be used with caution in those who have previously had cancer. Side effects include skin reactions such as redness, dryness, cracked skin, burning, stinging, peeling, itching, increased sensitivity to sunlight, sunburn, blisters and scarring. It may cause skin discolouration (i.e., blue or black discolouration or white patches or spots) that, in some cases, can be disfiguring. In laboratory animals, it has been associated with cancer after long-term exposure.
Tretinoin in topical format (applied to the skin) is a prescription drug used to treat acne. Topical tretinoin should not be used during pregnancy as it has been associated with birth defects. It should also not be used by those who are breastfeeding or by children under 12 years old, or by individuals who have inflamed or irritated skin, have a previous skin cancer or undiagnosed skin lesions, who are taking medicines that make their skin more sensitive to light, or who have an allergy to tretinoin. Tretinoin may cause pain, irritation, itchiness, redness, or swelling at the site of application. It may damage skin, change skin colour, and increase sensitivity to sunlight or tanning beds, causing sunburns. Using tretinoin in combination with hydroquinone may increase some of the side effects of tretinoin.
Betamethasone dipropionate, betamethasone valerate and clobetasol propionate are highly potent corticosteroid prescription drugs that are applied to the skin to treat inflammatory skin conditions. Side effects include skin irritation and, with prolonged use, skin weakening or deterioration. Adverse effects from using too much include decreased ability to fight infection, symptoms of adrenal gland suppression (i.e., low blood pressure, low blood sugar, weight loss, muscle pain, gastrointestinal problems and severe fatigue) or Cushing's syndrome (i.e., high blood pressure, high blood sugar, weight gain, muscle weakness, bone loss and severe fatigue), depending on how much has been absorbed. These drugs should not be used by pregnant or nursing people.
Ketoconazole is a prescription antifungal drug typically used to treat skin and scalp infections. It should be used only under the supervision of a health care professional. When applied to skin, common side effects include severe irritation, itchiness and stinging. Contact dermatitis (an itchy or painful rash) and painful allergic reactions have been reported more rarely. Ketoconazole should not be used by pregnant or breastfeeding people unless directed by a health care professional.
Neomycin sulfate is an antibiotic prescription drug and should be used only under the supervision of a health care professional. Side effects include allergic reactions that range from mild skin reactions (itching, rash and hives) to severe, life-threatening allergic reactions (anaphylaxis). Side effects such as damage to nerve tissue or the central nervous system, inner ear, and organs responsible for hearing and balance, and reduced kidney function have occurred in patients taking neomycin orally (by mouth) or when applied on the skin to open wounds or damaged skin. As well, when not used as directed, neomycin sulphate could increase the risk of infections resistant to neomycin or other antibiotics. Neomycin sulphate should not be used by pregnant or breastfeeding people unless directed by a health care professional.
Details
Media and public enquiries
Media enquiries
Health Canada
(613) 957-2983
media@hc-sc.gc.ca
Public enquiries
(613) 957-2991
1-866 225-0709
Get notified
Receive emails about new and updated recall and safety alerts.