Unauthorized skin lightening and skin treatment products may pose serious health risks
Summary
Stop using these products and consult your health care professional if you have health concerns. Report these or any unauthorized health products to Health Canada.
Affected products
| Photo | Product & (Promoted Use) | Hazard Identified | Company | Action Taken | Date Added |
|---|---|---|---|---|---|
 |
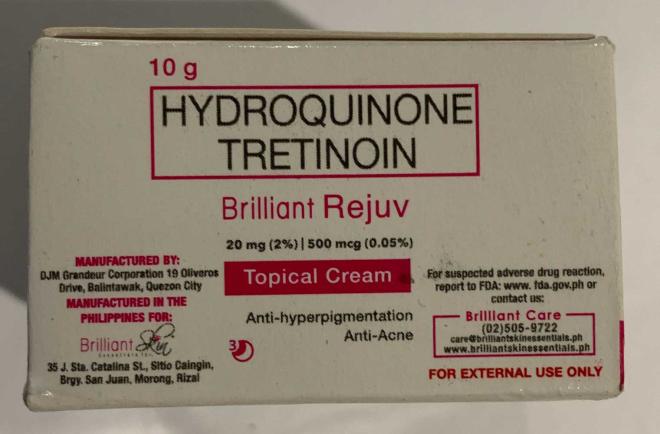
Brilliant Skin Essentials Brilliant Rejuv Set (Skin whitening) |
Labelled to contain hydroquinone and tretinoin | Hiraya Filo Store Victoria, B.C. |
Removed from the warehouse location and from online sale | 2024-08-07 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | Tokyo Beauty and Healthcare Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
Seized from retail location | 2024-03-27 |
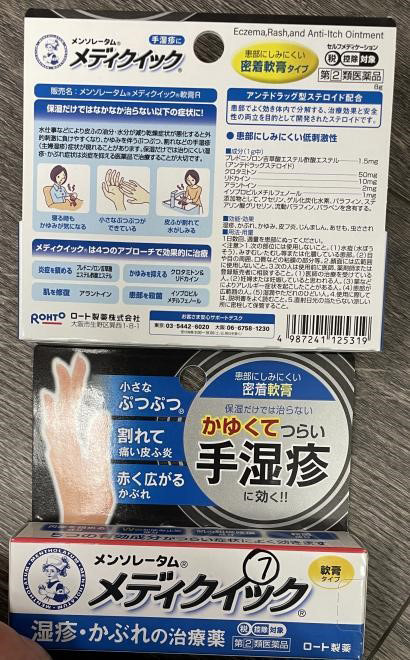 |
Mentholatum Mediquick Eczema Rash Anti-Itch Ointment (Skin conditions) |
Labelled to contain prednisolone valerate acetate | Tokyo Beauty and Healthcare Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
Seized from retail location | 2024-03-27 |
 |
Pair Acne Cream (Skin treatment) |
Labelled to contain ibuprofen piconol 3% | Tokyo Beauty and Healthcare Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
Seized from retail location | 2024-03-27 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | Jireh Trading & Marketing Inc. Richmond, B.C. |
Seized from warehouse and removed from online sale | 2023-12-27 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | Oceanbuy 2600 John St., Unit 107 Markham, ON |
Seized from retail location | 2023-12-13 |
 |
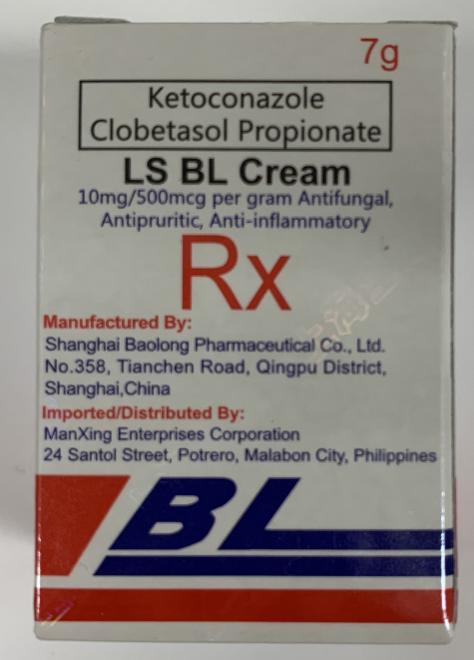
LS BL Cream (Skin treatment) |
Labelled to contain ketoconazole and clobetasol propionate | PAG-ASA Grocery Store 201 Grande Blvd #2, Cochrane, AB |
Seized from retail location | 2023-11-20 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | Foody Mart Warden 355 Bamburgh Circle, Scarborough, ON |
Seized from retail location | 2023-10-06 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | Foody World 8 William Kitchen Rd., Scarborough, ON |
Seized from retail location | 2023-10-06 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | Foody Mart Warden 355 Bamburgh Circle, Scarborough, ON |
Seized from retail location | 2023-10-06 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | Foody World 8 William Kitchen Rd., Scarborough, ON |
Seized from retail location | 2023-10-06 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | Kiyoko Beauty Aurora, ON |
Seized and removed from online sale | 2023-10-06 |
 |
Brilliant Skin Essentials Brilliant Rejuv (Skin whitening) |
Labelled to contain hydroquinone and tretinoin | Kolorete Canada 4944 Joyce Street Vancouver, B.C. |
Seized from retail location | 2023-09-13 |
 |
Dr. Alvin Rejuvenating Prime Depigmenting Agent Anti-acne (Skin treatment) |
Labelled to contain hydroquinone and tretinoin | Kolorete Canada 4944 Joyce Street Vancouver, B.C. |
Seized from retail location | 2023-09-13 |
 |
Dr. Alvin Rejuvenating Prime Topical Depigmenting Agent Anti-acne (Skin treatment) |
Labelled to contain hydroquinone and tretinoin | Kolorete Canada 4944 Joyce Street Vancouver, B.C. |
Seized from retail location | 2023-09-13 |
 |
Betnovate-N Cream (Skin treatment) |
Labelled to contain betamethasone valerate and neomycin | Thiara Supermarket 810 Nipissing Rd, Milton, ON |
Removed from sale | 2023-09-13 |
 |
Esapharma Lemonvate (Skin treatment) |
Labelled to contain clobetasol propionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
Esapharma Movate (Skin treatment) |
Labelled to contain clobetasol propionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
Funbact-A (Skin treatment) |
Labelled to contain betamethasone dipropionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
L'abidjanaise Crème (Skin whitening) |
Labelled to contain clobetasol propionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
Neoprosone Cream (Skin treatment) |
Labelled to contain clobetasol propionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
Neoprosone Gel (Skin treatment) |
Labelled to contain clobetasol propionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
Neutrotone Cream White Moon (Skin whitening) |
Labelled to contain clobetasol propionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
Tempovate Gel (Skin treatment) |
Labelled to contain clobetasol propionate | Genesis African Food Market 1181 Finch Ave W #16, North York, ON |
Seized from retail location | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 1 Promenade Cir Unit I, Thornhill, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 7070 Warden Ave., Markham, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 715 Central Pkwy W, Mississauga, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 224 Hunt Club Rd Unit A, Ottawa, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 9625 Yonge St, Richmond Hill, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 9255 Woodbine Ave, Markham, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 16005 Bayview Ave, Aurora, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 297 College St, Toronto, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Facial Foam (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 1800 Sheppard Ave E, North York, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 1 Promenade Cir Unit I, Thornhill, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 715 Central Pkwy W, Mississauga, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 224 Hunt Club Rd Unit A, Ottawa, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 9625 Yonge St, Richmond Hill, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 9255 Woodbine Ave, Markham, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 16005 Bayview Ave, Aurora, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 297 College St, Toronto, ON |
Removed from sale | 2023-09-13 |
 |
Hadalabo Pearl Barley Face Wash (Skin treatment) |
Labelled to contain aminocaproic acid | T&T Supermarket 1800 Sheppard Ave E, North York, ON |
Removed from sale | 2023-09-13 |
 |
Activ Clobe Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
BETACET-N Cream (Skin treatment) | Labelled to contain betamethasone dipropionate and neomycin | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Brilliant Skin Essentials Rejuvenating Facial Cream | Product tested by Health Canada and found to contain tretinoin and hydroquinone | Camrose Luxe Shoppe Camrose, AB |
Removed from the warehouse location and from online sale | 2023-04-19 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set | Labelled to contain hydroquinone and tretinoin | Dorton's Online shop.yyc Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream | Labelled to contain hydroquinone and tretinoin | Dorton's Online shop.yyc Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
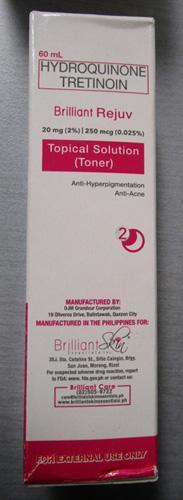
Brilliant Skin Essentials Brilliant Rejuv Topical Solution (Toner) |
Labelled to contain hydroquinone and tretinoin | Dorton's Online shop.yyc Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream | Labelled to contain hydroquinone and tretinoin | Camrose Luxe Shoppe Camrose, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Solution (Toner) | Labelled to contain hydroquinone and tretinoin | Camrose Luxe Shoppe Camrose, AB |
Removed from the warehouse location and from online sale | 2023-03-06 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream | Labelled to contain hydroquinone and tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Solution (Toner) | Labelled to contain hydroquinone and tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set | Labelled to contain hydroquinone and tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
 |
Brilliant Skin Essentials Rejuvenating Facial Toner | Product with similar packaging (previously removed from sale) was tested and found to contain tretinoin | Kausch International Goods Calgary, AB |
Removed from the warehouse location and from online sale | 2023-03-01 |
 |
Dermaglow Extra Whitening Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Dermaglow Extra Whitening Face Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Dermo Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Globatin antiMarks Complexion Lightening Cream (Skin whitening) | Labelled to contain clobetasol propionate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Medvate Cream (Skin whitening) | Labelled to contain betamethasone valerate | Alharameen Style & Decor Shop 255 28 Street SE #200 Calgary, AB |
Seized from retail location | 2023-05-18 |
 |
Betnovate-N 25 g (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Desi Mandi Store 1515 N Service Rd Burlington, ON |
Seized from retail location | 2023-05-18 |
 |
Betnovate-N 20 g (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Desi Mandi Store 1515 N Service Rd Burlington, ON |
Seized from retail location | 2023-05-18 |
 |
LS BL Cream (Skin treatment) | Labelled to contain ketoconazole and clobetasol propionate | Bags Clothing and Beyond Rosthern, SK |
Removed from sale | 2023-05-18 |
|
Beneks’ Fashion Fair Cream |
Labelled to contain clobetasol propionate |
De Chosen African Market |
Seized from retail location |
2023-07-27 |
|
|
Funbact-A |
Labelled to contain betamethasone dipropionate and neomycin sulphate |
De Chosen African Market |
Seized from retail location |
2023-07-27 |
|
|
Visita Plus |
Labelled to contain betamethasone dipropionate and neomycin sulphate |
De Chosen African Market |
Seized from retail location |
2023-07-27 |
|
|
Brilliant Skin Essentials Brilliant Rejuv Set |
Labelled to contain tretinoin |
Pinas-Sabay Canada and Y&Y Online Retail |
Seized from warehouse location and removed from online sale |
2023-07-27 |
|
|
Maxi-Peel Exfoliant Solution 1 |
Labelled to contain tretinoin |
Pinas-Sabay Canada and Y&Y Online Retail |
Seized from warehouse location and removed from online sale |
2023-07-27 |
|
|
Maxi-Peel Exfoliant Solution 2 |
Labelled to contain tretinoin |
Pinas-Sabay Canada and Y&Y Online Retail |
Seized from warehouse location and removed from online sale |
2023-07-27 |
|
|
Maxi-Peel Exfoliant Solution 3 |
Labelled to contain tretinoin |
Pinas-Sabay Canada and Y&Y Online Retail |
Seized from warehouse location and removed from online sale |
2023-07-27 |
|
|
RDL Babyface Solution |
Labelled to contain tretinoin |
Pinas-Sabay Canada and Y&Y Online Retail |
Seized from warehouse location and removed from online sale |
2023-07-27 |
|
 |
DGF Drugfield Penicillin Ointment (Skin treatment) | Labelled to contain penicillin potassium | African Foodways Market 1-282 St. Anne's Road Winnipeg, MB |
Seized from retail location | 2023-08-15 |
 |
Haloderm (Skin treatment) | Labelled to contain clobetasol propionate | African Foodways Market 1-282 St. Anne's Road Winnipeg, MB |
Seized from retail location | 2023-08-15 |
 |
Betamethasone Valerate and Neomycin Skin Cream (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Thiara Supermarket 3899 Trelawny Cir, Mississauga, ON |
Seized from retail location | 2023-08-15 |
 |
Betamethasone Valerate and Neomycin Skin Cream (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Thiara Supermarket 4265 Thomas Alton Blvd, Burlington, ON |
Seized from retail location | 2023-08-15 |
 |
Betnovate-N Cream (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Thiara Supermarket 3899 Trelawny Cir, Mississauga, ON |
Seized from retail location | 2023-08-15 |
 |
Betnovate-N Cream (Skin treatment) | Labelled to contain betamethasone valerate and neomycin | Thiara Supermarket 4265 Thomas Alton Blvd, Burlington, ON |
Seized from retail location | 2023-08-15 |
 |
MUHI Bug Repellant Cream (Skin treatment) | Labelled to contain dexamethasone | Kiokii and... 9350 Yonge St, Richmond Hill, ON |
Seized from retail location | 2023-08-15 |
 |
MUHI Bug Repellant Cream (Skin treatment) |
Labelled to contain dexamethasone | T&T Supermarket Unit 1115, 1800 Sheppard Ave. E North York, ON |
Seized from retail location | 2023-08-15 |
 |
Erythromycin Ointment (Skin treatment) |
Labelled to contain erythromycin | G&R Ginseng Trading Ltd. 1365-4540 No. 3 Rd. Richmond, B.C. |
Seized from retail location | 2023-08-15 |
 |
999 Ointment (yellow/orange) (Skin treatment) |
Labelled to contain ketoconazole | G&R Ginseng Trading Ltd. 1365-4540 No. 3 Rd. Richmond, B.C. |
Seized from retail location | 2023-08-15 |
 |
999 Ointment (yellow/orange) (Skin treatment) |
Labelled to contain ketoconazole | Yang Sheng Health Food Ltd. 1131-3779 Sexsmith Rd. Richmond, B.C. |
Seized from retail location | 2023-08-15 |
 |
999 Ointment (red) (Skin treatment) |
Labelled to contain dexamethasone | Yang Sheng Health Food Ltd. 1131-3779 Sexsmith Rd. Richmond, B.C. |
Seized from retail location | 2023-08-15 |
 |
Betnovate-N Cream (Skin treatment) |
Labelled to contain betamethasone valerate and neomycin | Global Choice Foods Ltd. 150 Rainbow Creek Drive Vaughan, ON |
Seized from retail location | 2023-08-23 |
 |
HADO LABO Gokujyun Hatomugi Blemish + Oil Control (Skin treatment) |
Labelled to contain aminocaproic acid | Sukoshi Mart 1800 Sheppard Ave E North York, ON |
Seized from retail location | 2023-08-23 |
 |
HADO LABO Gokujyun Trouble Care Skin Conditioner (Skin treatment) |
Labelled to contain aminocaproic acid | Sukoshi Mart 1800 Sheppard Ave E North York, ON |
Seized from retail location | 2023-08-23 |
 |
Acnesol Cream (Skin treatment) |
Labelled to contain tretinoin | Waange2020 Fashion 510 Sargent Ave, Unit 3, Winnipeg, MB |
Seized from retail location | 2024-12-02 |
 |
Defacto Cream (Skin treatment) |
Labelled to contain ketoconazole, clobetasal propionate and neomycin sulphate | Waange2020 Fashion 510 Sargent Ave, Unit 3, Winnipeg, MB |
Seized from retail location | 2024-12-02 |
 |
DHIIN DHIIN Cream Fast Action (Skin treatment) |
Labelled to contain clobetasal propionate | Waange2020 Fashion 510 Sargent Ave, Unit 3, Winnipeg, MB |
Seized from retail location | 2024-12-02 |
 |
La Dakaroise Cream Orange (Skin treatment) |
Labelled to contain clobetasal propionate | Waange2020 Fashion 510 Sargent Ave, Unit 3, Winnipeg, MB |
Seized from retail location | 2024-12-02 |
 |
Betnovate-N (Skin treatment) |
Labelled to contain betamethasone valerate and neomycin | All In One Wholesale Cash and Carry Ltd. 12815 85 Ave, Unit 107, Surrey, BC |
Seized from retail location | 2025-03-26 |
 |
Itch Guard + (Skin treatment) |
Labelled to contain terbinafine hydrochloride | Subzi Mandi Cash and Carry 1525 Bristol Rd, W, Mississauga, ON |
Seized from retail location | 2025-03-26 |
 |
Ring Guard (Skin treatment) |
Labelled to contain neomycin sulphate | Subzi Mandi Cash and Carry 1525 Bristol Rd, W, Mississauga, ON |
Seized from retail location | 2025-03-26 |
 |
Fluocinonide Cream (Skin treatment) |
Labelled to contain fluocinonide | South China Herbs Market Inc. 493 Dundas St W, Toronto, ON |
Seized from retail location | 2025-03-26 |
Issue
Health Canada is advising about unauthorized health products for lightening skin or treating skin conditions (such as eczema or psoriasis) that may pose serious health risks. The products are labelled to contain or have been tested and found to contain dangerous ingredients. Health Canada updates the table when it finds unauthorized health products for skin lightening. Links to previous tables with affected products are also available below.
Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality. Unauthorized health products can pose many health dangers, including:
- They may contain ingredients not listed on the label. This includes ingredients like prescription drugs, possibly at doses exceeding maximum recommended amounts. Prescription drugs should be taken only under the supervision of a health professional because they may cause serious side effects. Using a product that contains ingredients that the consumer is not aware of increases the chance of dangerous allergies and interactions with other medications and foods.
- The label may indicate a dangerous ingredient or combination of ingredients. For example, it could list a drug that should be available only by prescription from a health care professional, or a combination of ingredients that Health Canada does not permit because of serious health risks.
Health Canada maintains this page so that the public can easily identify products they may have purchased and take appropriate action. You are encouraged to check back regularly for updates. Advisories on safety issues involving other types of products are available in the recalls and safety alerts database.
What you should do
- Stop using the products listed in the table. Consult your health care professional if you have used these products and have health concerns, and for advice on which health products are best for you and your family.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check if products have been authorized for sale by searching Health Canada’s Drug Product Database and Licensed Natural Health Product Database.
- Report any health product adverse events or complaints to Health Canada.
- See these helpful links for additional information on buying health products safely:
What companies should know:
- Selling unauthorized health products in Canada is illegal.
- When Health Canada identifies unauthorized products that may pose serious health risks, Health Canada takes appropriate action to prevent further distribution and informs the public. This includes working with the Canada Border Services Agency to help prevent further importation of unauthorized products.
Additional information
Background
Aminocaproic acid is a prescription drug used to decrease bleeding in various clinical situations. Aminocaproic acid has not been approved for topical use in Canada. Exposure to aminocaproic acid on the skin may affect the skin, and the drug may be absorbed through the skin into the blood. Side effects may include a general feeling of discomfort, muscle aches and pain, kidney damage, seizures, low blood pressure, slow heart beat, blood clots, and swelling caused by fluid build-up.
Betamethasone dipropionate, betamethasone valerate and clobetasol propionate are highly potent corticosteroid prescription drugs that are applied to the skin to treat inflammatory skin conditions. Side effects include skin irritation and, with prolonged use, skin weakening or deterioration. Adverse effects from using too much include decreased ability to fight infection, symptoms of adrenal gland suppression (i.e., low blood pressure, low blood sugar, weight loss, muscle pain, gastrointestinal problems and severe fatigue) or Cushing's syndrome (i.e., high blood pressure, high blood sugar, weight gain, muscle weakness, bone loss and severe fatigue), depending on how much has been absorbed. These drugs should not be used by pregnant or nursing people.
Dexamethasone is a prescription corticosteroid drug available in Canada as tablets, injections, and eye ointments, and is used to treat inflammatory conditions or to suppress the immune system. Dexamethasone as an ointment is not approved as an anti-itch medication. Side effects for topical corticosteroids include skin atrophy (thin and fragile skin with reduced elasticity), skin blood vessel changes (e.g., spider veins), skin colour changes, stretch marks, swelling, dry skin, burning sensations, local irritation, rashes, redness, itching, thinning hair or excessive hair growth, infections and allergic reactions. Topical corticosteroids absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to experiencing these side effects. Systemic side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness and swelling. Dexamethasone should not be used by people who are allergic to dexamethasone or to any ingredient in the formulation, people who have systemic fungal infections, or people who have received live virus vaccines. Dexamethasone is generally not recommended for use during pregnancy.
Erythromycin is an antibacterial prescription drug used for the prevention and treatment of specific infections. In topical format (applied to the skin), it is used in combination with another drug to treat moderate acne. Possible reactions include local skin irritation (peeling, itching, burning sensation, redness or tenderness) or other skin changes (change in colour, oiliness or swelling), inflammation, or irritation of the face, eyes and nose. Symptoms of a severe allergic reaction include swelling of the face, mouth, throat, lips and/or eyes, difficulty breathing, burning and/or painful red eyes, eye tearing and skin reactions (rashes, blisters and hives). Misuse or overuse could lead to bacteria growth that could become resistant to the antibiotic erythromycin, which means that treatment may not work in the future.
Fluocinonide cream is a prescription corticosteroid drug used to treat inflammation and itching caused by skin conditions such as allergic reactions and eczema. It is a relatively strong corticosteroid cream. It can be absorbed through the skin, which may cause side effects throughout the body, especially when used over a large surface and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to systemic toxicity. Side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, swelling, or thinning of the skin. It is generally not recommended during pregnancy.
Hydroquinone is a prescription drug when it is greater than a 2% concentration and a natural health product at a concentration of 2% and under. It is used topically (applied to the skin) to lighten areas of darkened skin caused by different conditions (e.g., sun exposure, skin damage, pregnancy, medications or age). It should not be used by people who are allergic to hydroquinone or who are taking medicines that make their skin more sensitive to light. Hydroquinone is not recommended for pregnant or breastfeeding people, or for children. It should be used with caution in those who have previously had cancer. Side effects include skin reactions such as redness, dryness, cracked skin, burning, stinging, peeling, itching, increased sensitivity to sunlight, sunburn, blisters and scarring. It may cause skin discolouration (i.e., blue or black discolouration or white patches or spots) that, in some cases, can be disfiguring. In laboratory animals, it has been associated with cancer after long-term exposure.
Ibuprofen piconol 3% is a topical (applied to the skin) non-steroidal anti-inflammatory drug (NSAID) used to relieve burns. Health Canada has not approved any drugs containing ibuprofen for topical use. Ibuprofen absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time, or on damaged skin. Topical ibuprofen may cause serious side effects in people who are allergic to ibuprofen, aspirin or other NSAIDs, or who are asthmatic. Use of topical ibuprofen may also cause serious side effects such as stomach and intestinal bleeding, renal (kidney) dysfunction or failure, or cardiovascular dysfunction or failure in people who have problems with these organs. Topical ibuprofen can also cause serious side effects in people who are pregnant or breastfeeding, such as delayed and increased duration of labour.
Ketoconazole is a prescription antifungal drug typically used to treat skin and scalp infections. It should be used only under the supervision of a health care professional. When applied to skin, common side effects include severe irritation, itchiness and stinging. Contact dermatitis (an itchy or painful rash) and painful allergic reactions have been reported more rarely. Ketoconazole should not be used by pregnant or breastfeeding people unless directed by a health care professional.
Neomycin sulphate (or neomycin) is an antibiotic prescription drug and should be used only under the supervision of a health care professional. Side effects include allergic reactions that range from mild skin reactions (itching, rash and hives) to severe, life-threatening allergic reactions (anaphylaxis). Side effects such as damage to nerve tissue or the central nervous system, inner ear, and organs responsible for hearing and balance, and reduced kidney function have occurred in patients taking neomycin orally (by mouth) or when applied on the skin to open wounds or damaged skin. As well, when not used as directed, neomycin sulphate could increase the risk of infections resistant to neomycin or other antibiotics. Neomycin sulphate should not be used by pregnant or breastfeeding people unless directed by a health care professional.
Penicillin potassium is an antibacterial prescription drug used for the prevention and treatment of specific infections. Penicillin has not been approved for use in ointments for topical use in Canada. Allergic reactions to penicillin may include swelling of the face, mouth, throat, lips, eyes, difficulty breathing and skin reactions (itchiness, flushing, hives), abdominal distress (cramping, nausea, vomiting or diarrhea) and low blood pressure. Serious and occasionally fatal hypersensitivity (anaphylactoid) reactions have been reported in patients receiving penicillin. Severe skin reactions that may also affect other organs have been reported in association with penicillin. Prolonged use of antibiotics may lead to yeast or fungal overgrowth. Misuse or overuse of penicillin may lead to the growth of bacteria that will not be killed by penicillin (resistance). This means that penicillin may not work in the future.
Prednisolone valerate acetate is a prescription corticosteroid drug available in Canada as eye drops used to treat inflammation of several parts of the eye. It has not been approved for use in creams or ointments in Canada. Common side effects for topical corticosteroids include skin atrophy (thin and fragile skin with reduced elasticity), skin blood vessel changes (e.g., spider veins), change in skin color, stretch marks, swelling, dry skin, burning sensation, local irritation, rash, redness, itching, thinning hair or excessive hair growth, infections and allergic reactions. Topical corticosteroids absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to side effects. Systemic side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, and swelling. Prednisolone acetate should not be used in patients who are allergic to prednisolone acetate or to any ingredient in the formulation. Prednisolone acetate is not to be used in children and is not recommended for use during pregnancy or breastfeeding.
Terbinafine hydrochloride (HCl) cream is a prescription antifungal drug used to treat fungal and yeast infections of the skin. Side effects may include allergic reaction (hypersensitivity), flaking or peeling of the skin, itching, redness (skin rashes), pain, and skin irritation, including contact dermatitis (skin inflammation).
Tretinoin in topical format (applied to the skin) is a prescription drug used to treat acne. Topical tretinoin should not be used during pregnancy as it has been associated with birth defects. It should also not be used by those who are breastfeeding or by children under 12 years old, or by individuals who have inflamed or irritated skin, have a previous skin cancer or undiagnosed skin lesions, who are taking medicines that make their skin more sensitive to light, or who have an allergy to tretinoin. Tretinoin may cause pain, irritation, itchiness, redness, or swelling at the site of application. It may damage skin, change skin colour, and increase sensitivity to sunlight or tanning beds, causing sunburns. Using tretinoin in combination with hydroquinone may increase some of the side effects of tretinoin.
Details
Media and public enquiries
Media enquiries
Health Canada
(613) 957-2983
media@hc-sc.gc.ca
Public enquiries
(613) 957-2991
1-866 225-0709
Get notified
Receive emails about new and updated recall and safety alerts.