Unauthorized products may pose serious health risks (September 16, 2020: Part 2)
- Starting date:
- September 16, 2020
- Type of communication:
- Advisory
- Subcategory:
- Drugs, Natural health products
- Source of recall:
- Health Canada
- Issue:
- Unauthorized products, Important Safety Information
- Audience:
- General Public
- Identification number:
- RA-73935
Last updated: 2020-09-16
- Issue
- What you should do
- Background
- Affected products
- Related AWRs
- Media enquiries
- Public enquiries
- Images
Summary
- Product: Various unauthorized health products promoted for sexual enhancement, weight loss, as a workout aid, as "poppers," or for lightening skin or treating skin conditions (such as eczema or psoriasis).
- Issue: Products may contain dangerous ingredients such as prescription drugs that are not listed on the label, or the label may indicate a dangerous ingredient or combination of ingredients.
- What to do: Stop using these products and consult your healthcare professional if you have health concerns. Report these or any unauthorized health products to Health Canada.
Issue
Health Canada is advising Canadians about unauthorized health products that may pose serious health risks. The table below is updated when Health Canada finds unauthorized health products that are promoted for sexual enhancement, weight loss, as a workout aid, as "poppers," or for lightening skin or treating skin conditions (such as eczema or psoriasis). These products are labelled to contain or have been tested and found to contain dangerous ingredients. Links to previous tables with affected products are also available below.
Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality. Unauthorized health products can pose many health dangers, including:
- They may contain ingredients not listed on the label. This includes ingredients like prescription drugs, possibly at doses exceeding maximum recommended amounts. Prescription drugs should be taken only under the supervision of a health care professional because they may cause serious side effects. Using a product that contains ingredients that the consumer is not aware of increases the chance of dangerous allergies and interactions with other medications and foods.
- The label may indicate a dangerous ingredient or combination of ingredients. For example, it could list a drug that should be available only by prescription from a health care professional, or a combination of ingredients that Health Canada does not permit because of serious health risks.
Health Canada maintains this page so that Canadians can easily identify products they may have purchased and take appropriate action. Canadians are encouraged to check back regularly for updates. Advisories on safety issues involving other types of products are available in the recalls and safety alerts database.
What you should do
- Stop using the products listed below. Consult your health care professional if you have used these products and have health concerns, and for advice on which health products are best for you and your family.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check if products have been authorized for sale by searching Health Canada’s Drug Product Database and Licensed Natural Health Product Database.
- Report any health product adverse events or complaints to Health Canada.
- See the additional information on buying health products safely in the helpful links below.
What companies should know:
- Selling unauthorized health products in Canada is illegal.
- When Health Canada identifies unauthorized products that may pose serious health risks, Health Canada takes appropriate action to prevent further distribution and informs Canadians. This includes working with the Canada Border Services Agency to help prevent further importation of unauthorized products.
Background
Betamethasone dipropionate and clobetasol propionate are highly potent corticosteroid prescription drugs that are applied to the skin to treat inflammatory skin conditions. Side effects include skin irritation and, with prolonged use, skin weakening or deterioration. Adverse effects from using too much include decreased ability to fight infection, symptoms of adrenal gland suppression (i.e., low blood pressure, low blood sugar, weight loss, muscle pain, gastrointestinal problems and severe fatigue) or Cushing's syndrome (i.e., high blood pressure, high blood sugar, weight gain, muscle weakness, bone loss and severe fatigue) depending on how much has been absorbed. These drugs should not be used by pregnant or nursing women.
Cardarine (also known as GW501516 and GW1516), MK-677 and SR-9009 are not authorized in Canada for any use and may pose serious health risks. All clinical development activities to bring GW501516 to market were stopped when toxicities, including various cancers, were discovered following routine, long-term animal studies. The long-term effects in humans are unknown.
Dapoxetine is a drug used to treat premature ejaculation and is not authorized for sale in Canada. Side effects include fainting or loss of consciousness, dizziness, changes in blood pressure, blurred vision, seizures, headache, diarrhea and nausea. In particular, dapoxetine should not be used by individuals who have cardiac conditions, liver disease, a history of mania or bipolar disorder, or who are taking certain other medications, including monoamine oxidase inhibitors, antidepressants, and a number of other drugs and herbal products that can interact with dapoxetine.
Fluocinonide cream is a prescription corticosteroid drug used to treat inflammation and itching caused by skin conditions such as allergic reactions and eczema. It is a relatively strong corticosteroid cream. It can be absorbed through the skin, which may cause side effects throughout the body, especially when used over a large surface and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to systemic toxicity. Side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, swelling, or thinning of the skin. It is generally not recommended during pregnancy.
Hydroquinone for topical use at concentrations above 2% is a prescription drug used to lighten areas of darkened skin caused by different conditions (e.g., sun exposure, skin damage, pregnancy, medications or age). It should not be used by people who are allergic to hydroquinone or who are taking medicines that make their skin more sensitive to light. Hydroquinone is not recommended for pregnant or breastfeeding women, or children. It should be used with caution in those who have previously had cancer. Side effects include skin reactions such as redness, dryness, cracked skin, burning, stinging, peeling, itching, increased sensitivity to sunlight, sunburn, blisters and scarring. It may cause skin discolouration (i.e., blue or black discolouration or white patches or spots) that, in some cases, can be disfiguring. In laboratory animals, it has been associated with cancer after long-term exposure. As of June 30, 2019, products containing hydroquinone greater than 2% for topical use require a prescription from a healthcare practitioner to be sold in Canada. As part of this transition, several products exceeding 2% hydroquinone that were previously sold over the counter have been recalled in Canada.
L-Dopa, also known as levodopa, is a prescription drug that is combined with other drug ingredients in anti-Parkinson's medications. It should be used only under the supervision of a health care professional. Levodopa may interact with drugs prescribed for high blood pressure, and should not be used by women who are pregnant, who plan to become pregnant or who are breastfeeding. It should also not be taken by people with narrow angle glaucoma; untreated heart, liver, kidney, lung or hormonal diseases; a history of melanoma; or those who should not take drugs such as isoproterenol, amphetamines or epinephrine. Side effects requiring medical attention include: uncontrollable movements of the face, eyelids, mouth, tongue, neck, arms, hands, or legs; severe or persistent nausea or vomiting; an irregular heartbeat or fluttering in the chest; feeling lightheaded when standing quickly; or unusual changes in mood or behaviour.
Neomycin sulphate is an antibiotic prescription drug and should be used only under the supervision of a health care professional. Side effects include allergic reactions that range from mild skin reactions (itching, rash and hives) to severe, life-threatening allergic reactions (anaphylaxis). Side effectsâsuch as damage to nerve tissue or the central nervous system, inner ear, and organs responsible for hearing and balance, and reduced kidney functionâhave occurred in patients taking neomycin orally (by mouth) or when applied on the skin to open wounds or damaged skin. As well, when not used as directed, neomycin sulphate could increase the risk of infections resistant to neomycin or other antibiotics. Neomycin sulphate should not be used by pregnant or breastfeeding women unless directed by a healthcare professional.
Rauwolfia (also known as rauvolfia) is a prescription drug that can be used to treat high blood pressure (hypertension). People with heart conditions or psychiatric disorders should receive close medical supervision when using it as it can make certain conditions, such as depression, worse. Common side effects include tiredness, sleepiness, depression, nasal obstruction and breathlessness. Rauwolfia should not be used by children, or pregnant or nursing women.
Selective Androgen Receptor Modulators (SARMs) are drugs that are not authorized in Canada for any use and have not been reviewed by Health Canada for safety, effectiveness and quality. The use of bodybuilding products that contain SARMs can pose serious health risks such as heart attack, stroke and liver damage. The long-term effects on the body are unknown. Examples include andarine, ostarine (MK 2866), ligandrol (LDG-4033) myostine (YK-11), and testolone (RAD-140).
Sildenafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g. nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss. Desmethyl carbodenafil and dithiodesmethyl carbodenafil are unauthorized substances that are similar to sildenafil and may pose similar health risks.
Tadalafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) since it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Vitamin A is considered a prescription drug when taken orally (by mouth) at doses of more than 10,000 International Units (IU) per day. Too much vitamin A can be harmful and cause nausea and vomiting, diarrhea, headache, irritability, sleep disturbances, fever, loss of appetite and weight loss. Too much vitamin A can also cause bone thinning, peeling of the skin, and inflammation of the lips, inside of the mouth, tongue or gums. Women who are or who might become pregnant should talk to their doctor before taking vitamin A. Taking too much of vitamin A during pregnancy can cause serious birth defects.
Vitamin D is considered a prescription drug when taken orally (by mouth) at doses of more than 1,000 International Units (IU) per day. Too much vitamin D can lead to vitamin D "intoxication," which can cause weakness, fatigue, drowsiness, headache, lack of appetite, dry mouth, metallic taste, nausea, vomiting, vertigo, ringing in the ears, lack of coordination, and muscle weakness. Pregnant women in particular should not take vitamin D exceeding the daily tolerable upper intake level for adults (4,000 IU).
Vitamin K is considered a prescription drug when taken orally (by mouth) at doses of more than 120ug per day. Vitamin K is known to reduce the effectiveness of blood thinners such as warfarin. Individuals taking blood thinners need to talk to their doctor before taking vitamin K. Additionally, vitamin K can cause symptoms of heart problems such as chest pain, pale skin, low blood pressure and elevated heart rate. It has also been reported to cause decreased appetite, dizziness, sweating, yellow eyes or skin, anaphylaxis (a life-threatening allergic reaction), blue skin or lips, shortness of breath, and skin changes such as rash, redness, hives or itching.
Yohimbine is a prescription drug and should be used only under the supervision of a health care professional. Yohimbine is derived from yohimbe, a bark extract. The use of yohimbine or yohimbe may result in serious adverse reactions particularly in people with high blood pressure, or heart, kidney or liver disease. Side effects include increased blood pressure and heart rate, anxiety, dizziness, tremors, headache, nausea and sleep disorders. It should not be used by children, or pregnant or nursing women.
Affected products
See table Part 1 and Part 2. Links to previous tables with affected products are also available below.
Product description
| Photo | Product & Promoted Use | Hazard Identified | Company | Action Taken | Date Added |
|---|---|---|---|---|---|
|
Benek's Fashion Fair Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Benek's Fashion Fair Gel Plus Skin lightening |
Labelled to contain betamethasone dipropionate 0.065g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Benek's New Hot Movate Gel Skin treatment |
Labelled to contain betamethasone dipropionate 0.075g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Civic Cream Skin treatment |
Labelled to contain clobetasol propionate 0.05g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Esapharma Lemonvate Cream Skin treatment |
Labelled to contain clobetasol propionate 0.05g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Esapharma Movate Cream Skin treatment |
Labelled to contain clobetasol propionate 0.05g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
H2O Jours Natural Gel Plus Skin lightening |
Labelled to contain betamethasone dipropionate 0.05% |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Haloderm Skin treatment |
Labelled to contain clobetasol proprionate 0.05% |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Janet Papaya Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Mekako Lightening Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Nadinola Extra Strength Discoloration Fade Cream Skin lightening |
Labelled to contain hydroquinone 3% |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Othine Skin Discoloration Fade Cream Skin lightening |
Labelled to contain hydroquinone 3% |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Top Gel Plus Skin treatment |
Labelled to contain fluocinonide 0.025g, neomycin sulphate 0.05g |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
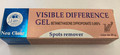
|
Visible Difference Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Visible Difference Gel Skin lightening |
Labelled to contain betamethasone dipropionate 0.065% |
Beauty Collection Inc. 11-2058 Ellesmere Rd. Scarborough, ON |
Seized from the retail location | September 16, 2020 | |
|
Beneks Hot Movate Gel Skin Skin treatment |
Labelled to contain betamethasone dipropionate |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Dermovate Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Esapharma Movate cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Janet Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Janet Gel Plus Aloé Véra Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Janet Papaya Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
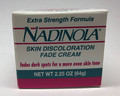
|
Nadinola Extra Strength Formula Skin Discolouration Fade Cream Skin lightening |
Labelled to contain hydroquinone 3% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Nature Secrète with the Oil Argon cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Naturel Cream Aloe Vera Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Naturel Lemon Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Naturel Papaya Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
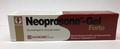
|
Neoprosone-Gel Forte Skin lightening |
Labelled to contain betamethasone dipropionate 0.05% and neomycin sulphate 0.5% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
SURFAZ-SN Triple Action Cream Skin treatment |
Labelled to contain betamethasone dipropionate 0.05% and neomycin sulphate 0.5% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
White Express Fast Action Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Marilyn's Beauty Supply 1824 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Beneks Fashion Fair Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Beneks' Fashion Fair Gel Plus Skin treatment |
Labelled to contain betamethasone dipropionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Dr White Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Funbact-A Triple Action Cream Skin treatment |
Labelled to contain betamethasone dipropionate 0.05% and neomycin sulphate 0.5% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Lemonvate Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Naturel Gel Plus Skin lightening |
Labelled to contain betamethasone dipropionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Naturel Papaya Cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Nature Secrète with the Oil Argon cream Skin lightening |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Othine Skin Bleach Skin lightening |
Labelled to contain hydroquinone 3% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Pop Popular Cream Skin treatment |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
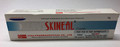
|
Skineal Cream Skin treatment |
Labelled to contain clobetasol propionate 0.25mg and neomycin sulfate 5000IU |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
So Nice Cream Skin treatment |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 | |
|
Topifram Skin treatment |
Labelled to contain clobetasol propionate 0.05% |
Caribbean Island Food Market, 3432 Weston Rd. Toronto, ON |
Seized from the retail location | September 16, 2020 |
Related AWRs
Unauthorized products may pose serious health risks (November 17, 2017 to May 23, 2018)
2017-11-17 | Health products
Unauthorized products may pose serious health risks (June 5, 2018 to November 7, 2018)
2018-06-05 | Health products
Unauthorized products may pose serious health risks (December 10, 2018 to April 17, 2019)
2018-12-10 | Health products
Unauthorized products may pose serious health risks (May 17, 2019 - Part 1)
2019-05-17 | Health products
Unauthorized products may pose serious health risks (May 17, 2019 - Part 2)
2019-05-17 | Health products
Unauthorized products may pose serious health risks (June 11, 2019 to July 26, 2019)
2019-06-11 | Health products
Unauthorized products may pose serious health risks (August 12, 2019 to September 18, 2019)
2019-08-12 | Health products
Unauthorized products may pose serious health risks (October 2, 2019 to November 19, 2019)
2019-10-02 | Health products
Unauthorized products may pose serious health risks (December 9, 2019 to January, 17 2020)
2019-12-09 | Health products
Unauthorized products may pose serious health risks (February 10, 2020 to March 13, 2020)
2020-02-10 | Health products
Unauthorized products may pose serious health risks (October 21, 2020)
2020-10-21 | Health products
Unauthorized products may pose serious health risks (Part 1) (February 18, 2021)
2021-02-18 | Health products
Unauthorized products may pose serious health risks (Part 2) (February 18, 2021)
2021-02-18 | Health products
Unauthorized products may pose serious health risks (November 25, 2020)
2020-11-25 | Health products
Media enquiries
Health Canada
(613) 957-2983
hc.media.sc@canada.ca
Public enquiries
(613) 957-2991
1-866 225-0709
hcinfo.infosc@canada.ca
Images
Select thumbnail to enlarge - opens in a new window