Unauthorized products may pose serious health risks (December 9, 2019 to January, 17 2020)
- Starting date:
- December 9, 2019
- Posting date:
- January 17, 2020
- Type of communication:
- Advisory
- Subcategory:
- Drugs, Natural health products
- Source of recall:
- Health Canada
- Issue:
- Unauthorized products, Important Safety Information
- Audience:
- General Public
- Identification number:
- RA-71843
Last updated: 2020-02-10
- Issue
- What you should do
- Background
- Affected products
- Related AWRs
- Media enquiries
- Public enquiries
- Images
Summary
- Product: Various unauthorized health products promoted for sexual enhancement, weight loss, as a workout aid, or as "poppers".
- Issue: Products may contain dangerous ingredients such as prescription drugs that are not listed on the label, or the label may indicate a dangerous ingredient or combination of ingredients.
- What to do: Stop using these products and consult your healthcare professional if you have health concerns. Report these or any unauthorized health products to Health Canada.
Issue
Health Canada is advising Canadians about unauthorized health products that may pose serious health risks. The table below is updated when Health Canada finds unauthorized health products that are promoted for sexual enhancement, weight loss, as a workout aid, or as "poppers," and that are labelled to contain or have been tested and found to contain dangerous ingredients. Links to previous tables with affected products are also available below.
Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality. Unauthorized health products can pose many health dangers, including:
- They may contain ingredients not listed on the label. This includes ingredients like prescription drugs, possibly at doses exceeding maximum recommended amounts. Prescription drugs should be taken only under the supervision of a health care professional because they may cause serious side effects. Using a product that contains ingredients that the consumer is not aware of increases the chance of dangerous allergies and interactions with other medications and foods.
- The label may indicate a dangerous ingredient or combination of ingredients. For example, it could list a drug that should be available only by prescription from a health care professional, or a combination of ingredients that Health Canada does not permit because of serious health risks.
Health Canada maintains this page so that Canadians can easily identify products they may have purchased and take appropriate action. Canadians are encouraged to check back regularly for updates. Advisories on safety issues involving other types of products are available in the recalls and safety alerts database.
What you should do
- Stop using the products listed below. Consult your health care professional if you have used these products and have health concerns, and for advice on which health products are best for you and your family.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check if products have been authorized for sale by searching Health Canada’s Drug Product Database and Licensed Natural Health Product Database.
- Report any health product adverse events or complaints to Health Canada.
- See the additional information on buying health products safely in the helpful links below.
What companies should know:
- Selling unauthorized health products in Canada is illegal.
- When Health Canada identifies unauthorized products that may pose serious health risks, Health Canada takes appropriate action to prevent further distribution and informs Canadians. This includes working with the Canada Border Services Agency to help prevent further importation of unauthorized products.
Background
Cardarine (also known as GW501516 and GW1516), MK-677 and SR-9009 are not authorized in Canada for any use and may pose serious health risks. All clinical development all activities to bring GW501516 to market were stopped when toxicities, including various cancers, were discovered following routine, long-term animal studies. The long-term effects in humans are unknown.
"Poppers" is a slang term for products that contain alkyl nitrites. Despite being labelled for various uses such as leather cleaners, room odourizers or liquid incense, these products are inhaled or ingested by consumers for recreational purposes. Alkyl nitrites, such as amyl nitrite, butyl nitrite and isobutyl nitrite, are prescription drugs and should be used only under the supervision of a healthcare professional. Products containing alkyl nitrites may pose serious risks, including death, depending on the amount used, how frequently they are used and how long they are used for, as well as the person's health and the other medications they may be taking. Since it is difficult to control how much is inhaled, people can accidentally overdose. Swallowing these products can lead to serious medical complications and may be fatal. People with certain medical conditions (including recent head trauma, bleeding into the head, glaucoma, or heart disease) and those taking certain medications (particularly drugs used to treat erectile dysfunction, and other drugs such as high blood pressure medications, certain migraine drugs, and high doses of aspirin) or illicit drugs are at particular risk.
Rauwolfia (also known as rauvolfia) is a prescription drug that can be used to treat high blood pressure (hypertension). People with heart conditions or psychiatric disorders should receive close medical supervision when using it as it can make certain conditions, such as depression, worse. Common side effects include tiredness, sleepiness, depression, nasal obstruction and breathlessness. Rauwolfia should not be used by children, or pregnant or nursing women.
Selective Androgen Receptor Modulators (SARMs) are drugs that are not authorized in Canada for any use and have not been reviewed by Health Canada for safety, effectiveness and quality. The use of bodybuilding products that contain SARMs can pose serious health risks such as heart attack, stroke and liver damage. The long-term effects on the body are unknown. Examples include andarine, ostarine (MK 2866), ligandrol (LDG-4033) myostine (YK-11), and testolone (RAD-140).
Sildenafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss. Hydroxythiohomosildenafil and thiodimethylsildenafil are unauthorized substances that are similar to sildenafil and may pose similar health risks.
Tadalafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) since it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss. Aminotadalafil is an unauthorized substance that is similar to tadalafil and may pose similar health risks.
Yohimbine is a prescription drug and should be used only under the supervision of a health care professional. Yohimbine is derived from yohimbe, a bark extract. The use of yohimbine or yohimbe may result in serious adverse reactions particularly in people with high blood pressure, or heart, kidney or liver disease. Side effects include increased blood pressure and heart rate, anxiety, dizziness, tremors, headache, nausea and sleep disorders. It should not be used by children, or pregnant or nursing women.
Affected products
Links to previous tables with affected products are also available below.
Product description
| Photo |
Product & Promoted Use |
Hazard Identified | Company | Action Taken | Date Added |
|---|---|---|---|---|---|
|
Spanish Fly Sex Liquid Wild Strawberry |
Product with similar packaging (previously seized) was labelled to contain yohimbe |
Balout Supermarket |
Seized from the retail location |
January 17, 2020 |
|
|
Vigour 800 |
Product with same name was tested by the Australian Therapeutic Goods Administration and found to contain sildenafil |
Balout Supermarket |
Seized from the retail location |
January 17, 2020 |
|
|
Zhen Gongfu |
Product with similar packaging (previously seized) was tested and found to contain sildenafil |
Balout Supermarket |
Seized from the retail location |
January 17, 2020 |
|
|
Zhen Gongfu |
Product with similar packaging (previously seized) was tested and found to contain sildenafil |
Pars Foods Inc. |
Seized from the retail location |
January 17, 2020 |
|
|
777K |
Labelled to contain yohimbe |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Bl4ck 4K |
Product with similar packaging (previously seized) was tested and found to contain sildenafil |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Boss Lion 9000 |
Product with similar packaging tested by the U.S. Food and Drug Administration |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Gold Lion Gold Label 3000mg |
Product with similar packaging (previously seized) was tested and found to contain hydroxythiohomosildenafil |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Green Mamba |
Labelled to contain yohimbe |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Poseidon Platinum 3500 |
Product with similar packaging (previously seized) was tested and found to contain sildenafil |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
ResERECTION! |
Product with similar packaging (previously seized) was tested and found to contain |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Rodeo Fantasy Triple Maximum |
Labelled to contain yohimbe |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Titanium 4000 |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Ultimate 3500 |
Product with similar packaging (previously seized) was tested and found to contain thiodimethylsildenafil |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Jungle Juice Platinum |
Product with similar packaging (previously seized) was labelled to contain alkyl nitrites |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Jungle Juice Platinum Black |
Product with similar packaging (previously seized) was labelled to contain alkyl nitrites |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Rush |
Product with similar packaging (previously seized) was labelled to contain alkyl nitrites |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Super Rush |
Product with similar packaging (previously seized) was labelled to contain alkyl nitrites |
Lil’ Devil Adult Video & Toys |
Seized from the retail location |
January 9, 2020 |
|
|
Alien Power Platinum 11000 |
Product with similar packaging (previously seized) was tested and found to contain tadalafil |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
|
|
Master Zone 1500 |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
ResERECTION! |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
|
ResERECTION! (Bottle) |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
|
Rhino |
Product with similar packaging (previously seized) was tested and found to contain aminotadalafil |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
|
Super Panther 7K |
Labelled to contain yohimbe |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
|
Triple Green |
Labelled to contain yohimbe |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
|
VIP GO Rhino Gold 69K |
Labelled to contain yohimbe bark extract |
Forbidden Pleasures |
Seized from the retail location |
January 9, 2020 |
|
|
7k |
Labelled to contain yohimbe |
Time Square Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
3800 Hard Rock |
Labelled to contain yohimbe |
Time Square Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
Lollipop |
Product with similar packaging (previously seized) was tested and found to contain sildenafil, tadalafil and yohimbine |
St. James Town Gifts & Variety |
Seized from the retail location |
January 9, 2020 |
|
|
3800 Hard Rock |
Labelled to contain yohimbe |
St. James Town Gifts & Variety |
Seized from the retail location |
January 9, 2020 |
|
|
Black Panther #1 |
Labelled to contain yohimbe |
St. James Town Gifts & Variety |
Seized from the retail location |
January 9, 2020 |
|
|
Rhino 7 Platinum 5000 |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and yohimbine |
St. James Town Gifts & Variety |
Seized from the retail location |
January 9, 2020 |
|
|
Super Panther 7k |
Labelled to contain yohimbe |
St. James Town Gifts & Variety |
Seized from the retail location |
January 9, 2020 |
|
|
XXLANT 3000 |
Labelled to contain yohimbe |
St. James Town Gifts & Variety |
Seized from the retail location |
January 9, 2020 |
|
|
Black Panther 200k |
Product with similar packaging (previously seized) was labelled to contain yohimbe |
St. James Town Gifts & Variety |
Seized from the retail location |
January 9, 2020 |
|
|
3800 Hard Rock |
Labelled to contain yohimbe |
One More Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
7k |
Labelled to contain yohimbe |
One More Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
Black Panther #1 |
Labelled to contain yohimbe |
One More Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
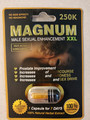
Magnum Male Sexual enhancement XXL |
Product with similar packaging tested by Australia’s Therapeutic Goods Administration |
One More Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
Rhino 25 Platinum 25000 |
Product with similar packaging (previously seized) was labelled to contain yohimbe |
One More Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
Stiff Rox |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil |
One More Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
XXLANT 3000 |
Labelled to contain yohimbe |
One More Convenience |
Seized from the retail location |
January 9, 2020 |
|
|
3800 Hard Rock |
Labelled to contain yohimbe |
Corner Convenience Store |
Seized from the retail location |
January 9, 2020 |
|
|
Black Mamba |
Labelled to contain yohimbe |
Corner Convenience Store |
Seized from the retail location |
January 9, 2020 |
|
|
Ginseng Red 2000 |
Product with similar packaging (previously seized) was tested and found to contain sildenafil |
Corner Convenience Store |
Seized from the retail location |
January 9, 2020 |
|
|
Black Stallion Platinum 30k |
Labelled to contain yohimbe |
Corner Convenience Store |
Seized from the retail location |
January 9, 2020 |
|
|
Premium Pro Power 3500 |
Labelled to contain yohimbe |
Corner Convenience Store |
Seized from the retail location |
January 9, 2020 |
|
|
Rhino 7 Platinum 5000 |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and yohimbine |
Corner Convenience Store |
Seized from the retail location |
January 9, 2020 |
|
|
Gold Lion Gold Label 3000mg |
Product tested by Health Canada and found to contain hydroxythiohomosildenafil |
Hespeler Road Adult Superstore |
Seized from the retail location |
January 9, 2020 |
|
|
Jaguar 30000 |
Product tested by Health Canada and found to contain tadalafil |
Hespeler Road Adult Superstore |
Seized from the retail location |
January 9, 2020 |
|
|
MV7 Days Power Gummy |
Product tested by Health Canada and found to contain tadalafil |
Hespeler Road Adult Superstore |
Seized from the retail location |
January 9, 2020 |
|
|
MYO-CARD Workout supplement |
Labelled to contain cardarine GW-501516 |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-HER Workout supplement |
Labelled to contain ostarine MK-2866 |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-HGH Workout supplement |
Labelled to contain MK-677 |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-LGD Workout supplement |
Labelled to contain ligandrol LGD-4033 |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-S4 Workout supplement |
Labelled to contain andarine |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-STA Workout supplement |
Labelled to contain ostarine MK-2866 |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-RAD Workout supplement |
Labelled to contain RAD 140 testolone |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-TKO Total Knock-Out Workout supplement |
Labelled to contain ostarine MK-2866 |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
MYO-YK Workout supplement |
Labelled to contain YK-11 myostine |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 | |
|
Psychotic Workout supplement |
Labelled to contain rauwolfia vomitoria extract (std. min. 90% alpha yohimbine) |
Lethbridge Supplements & Vitamins 1406 3rd Avenue South, Lethbridge, AB |
Seized from the retail location | December 9, 2019 |
Related AWRs
Unauthorized products may pose serious health risks (November 17, 2017 to May 23, 2018)
2017-11-17 | Health products
Unauthorized products may pose serious health risks (June 5, 2018 to November 7, 2018)
2018-06-05 | Health products
Unauthorized products may pose serious health risks (December 10, 2018 to April 17, 2019)
2018-12-10 | Health products
Unauthorized products may pose serious health risks (May 17, 2019 - Part 1)
2019-05-17 | Health products
Unauthorized products may pose serious health risks (May 17, 2019 - Part 2)
2019-05-17 | Health products
Unauthorized products may pose serious health risks (June 11, 2019 to July 26, 2019)
2019-06-11 | Health products
Unauthorized products may pose serious health risks (August 12, 2019 to September 18, 2019)
2019-08-12 | Health products
Unauthorized products may pose serious health risks (October 2, 2019 to November 19, 2019)
2019-10-02 | Health products
Unauthorized products may pose serious health risks (September 16, 2020: Part 1)
2020-09-16 | Health products
Unauthorized products may pose serious health risks (September 16, 2020: Part 2)
2020-09-16 | Health products
Unauthorized products may pose serious health risks (February 10, 2020 to March 13, 2020)
2020-02-10 | Health products
Unauthorized products may pose serious health risks (October 21, 2020)
2020-10-21 | Health products
Unauthorized products may pose serious health risks (Part 1) (February 18, 2021)
2021-02-18 | Health products
Unauthorized products may pose serious health risks (Part 2) (February 18, 2021)
2021-02-18 | Health products
Unauthorized products may pose serious health risks (November 25, 2020)
2020-11-25 | Health products
Media enquiries
Health Canada
(613) 957-2983
hc.media.sc@canada.ca
Public enquiries
(613) 957-2991
1-866 225-0709
hcinfo.infosc@canada.ca
Images
Select thumbnail to enlarge - opens in a new window