Unauthorized products may pose serious health risks (June 16, 2022 to February 10, 2023)
Summary
Stop using these products and consult your health care professional if you have health concerns. Report these or any unauthorized health products to Health Canada.
Affected products
| Photo | Product & Promoted Use | Hazard Identified | Company | Action Taken | Date Added |
|---|---|---|---|---|---|
 |
Tretinoin Cream Anti-Acne Anti-Wrinkle Youth Renewal Night Cream |
Labeled to contain tretinoin |
Beauty Lounge Canada |
Removed from the warehouse location and from online sale |
2023-01-25 |
 |
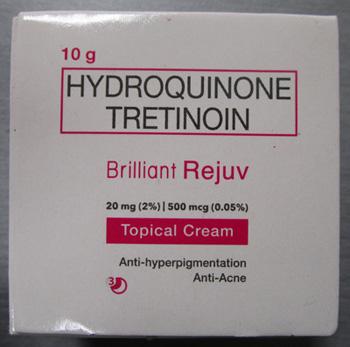
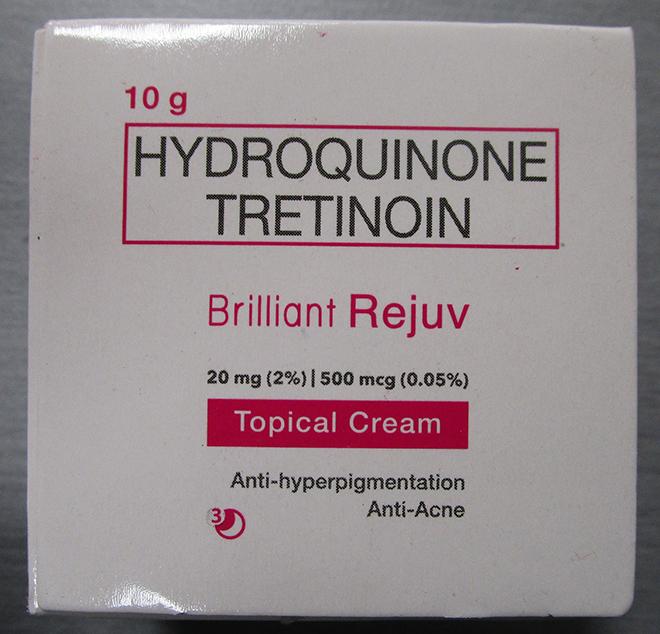
Brilliant Skin Essentials Brilliant Rejuv Topical Cream |
Labelled to contain hydroquinone and tretinoin |
Project Glo Up / Glowify |
Seized from the warehouse location and removed from online sale |
2023-01-25 |
 |
Brilliant Skin Essentials Topical Solution (Toner) |
Labelled to contain hydroquinone and tretinoin |
Project Glo Up / Glowify |
Seized from the warehouse location and removed from online sale |
2023-01-25 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set |
Labelled to contain hydroquinone and tretinoin |
Project Glo Up / Glowify |
Seized from the warehouse location and removed from online sale |
2023-01-25 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set |
Labelled to contain hydroquinone and tretinoin |
Beauty Haven Canada |
Seized from the warehouse location and removed from online sale |
2022-12-28 |
 |
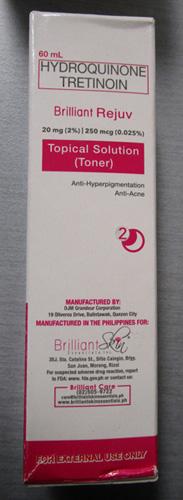
Brilliant Skin Essentials Brilliant Rejuv Topical Solution |
Labelled to contain hydroquinone and tretinoin |
Beauty Haven Canada |
Seized from the warehouse location and removed from online sale |
2022-12-28 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream |
Labelled to contain hydroquinone and tretinoin |
Beauty Haven Canada |
Seized from the warehouse location and removed from online sale |
2022-12-28 |
 |
Arize (capsules) Sexual enhancement |
Product tested by Health Canada and found to contain tadalafil or nortadalafil | Les Folies du Coeur 7580 boul. Henri Bourassa Quebec, QC |
Seized from the retail location | 2022-12-14 |
 |
Pink Pussycat Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain hydroxythiohomosildenafil |
Aren't We Naughty Locations: 80 Bramwin Court 1755 Pickering Parkway 4040 Highway 7, unit 2 |
Seized from the retail location | 2022-12-14 |
 |
Titanium 4000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil |
Aren't We Naughty Locations: 80 Bramwin Court 1755 Pickering Parkway |
Seized from the retail location | 2022-12-14 |
 |
Brilliant Skin Rejuvenating Facial Cream Skin lightening |
Product tested by Health Canada and found to contain tretinoin | Glamouroza E-retail Red Deer, AB |
Removed from sale | 2022-10-17 |
 |
Brilliant Skin Rejuvenating Facial Toner Skin lightening |
Product tested by Health Canada and found to contain tretinoin | Glamouroza E-retail Red Deer, AB |
Removed from sale | 2022-10-17 |
 |
Black Panther Power Gummy Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil and tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-10-12 |
 |
Gold Rhino Super Long Lasting Extreme 285k Sexual enhancement |
Product tested by Health Canada and found to contain tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-10-12 |
 |
Libigrow Platinum 10,000 Sexual enhancement |
Product tested by Health Canada and found to contain prasterone | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-10-12 |
 |
Plus power Love 20,000 Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-10-12 |
 |
Rhino Super Long Lasting 750k Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil and tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-10-12 |
 |
Rhino Super Long Lasting Premium 500k Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-10-12 |
 |
Maxi-Peel Exfoliant Solution 2 Skin lightening |
Labelled to contain tretinoin and 2% hydroquinone | Glamouroza E-retail Red Deer, AB |
Removed from sale | 2022-09-27 |
 |
Rhino 69 Extreme 500k Sexual enhancement | Product tested by Health Canada and found to contain prasterone | Esso Gas Station 89 Lindsay Street S. Lindsay, ON |
Seized from the retail location | 2022-09-27 |
| Blue 6K Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 | |
 |
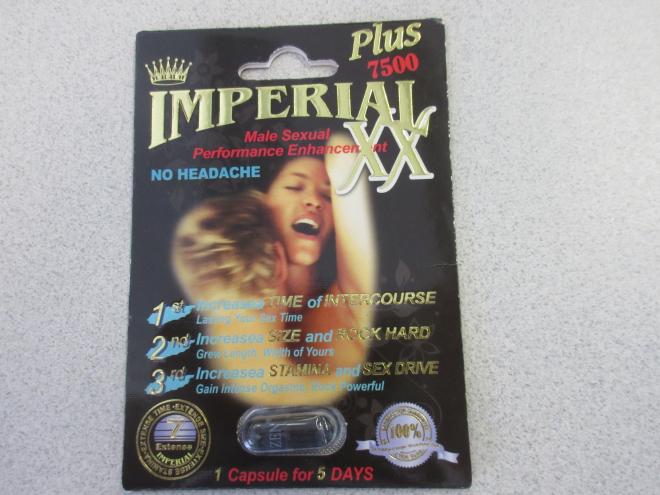
Imperial XX Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil and tadalafil |
Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 |
 |
One Million Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 |
 |
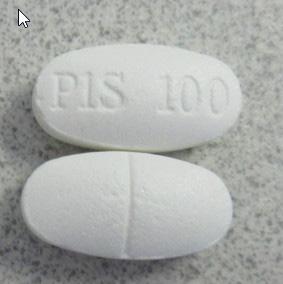
P1S 100 Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 |
 |
Rhino 17 Plus Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 |
 |
Rhino 69 Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 |
 |
Rhino 99 Extreme 188K Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 |
 |
Rhino Platinum 8000 Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-08-31 |
 |
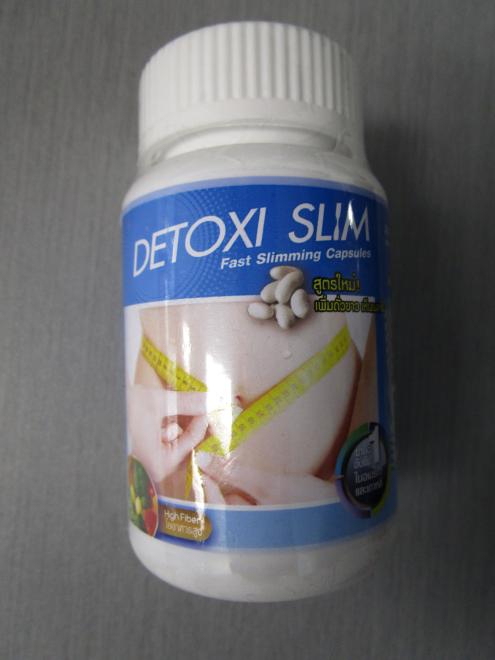
Detoxi Slim Weight loss |
Product tested by Health Canada and found to contain sibutramine | Jedpar Health and Beauty Lethbridge, AB |
Seized from the warehouse location and removed from online sale | 2022-08-31 |
 |
Idol Slim Coffee Weight loss |
Product tested by Health Canada and found to contain lorcaserin | Jedpar Health and Beauty Lethbridge, AB |
Seized from the warehouse location and removed from online sale | 2022-08-31 |
 |
Lida Shake Weight loss |
Product tested by Health Canada and found to contain lorcaserin | Jedpar Health and Beauty Lethbridge, AB |
Seized from the warehouse location and removed from online sale | 2022-08-31 |
 |
Max Slim Weight loss |
Product tested by Health Canada and found to contain sibutramine | Jedpar Health and Beauty Lethbridge, AB |
Seized from the warehouse location and removed from online sale | 2022-08-31 |
 |
Jungle Juice Platinum Black Poppers |
Product tested by Health Canada and found to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway Etobicoke, ON |
Seized from the retail location | 2022-08-31 |
 |
Kidney Boost Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Wiggles Adult Video 980 The Queensway Etobicoke, ON |
Seized from the retail location | 2022-08-31 |
 |
Super Rush Power Pack Poppers |
Product tested by Health Canada and found to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway Etobicoke, ON |
Seized from the retail location | 2022-08-31 |
 |
Titanium 4000 (tablet) Sexual enhancement |
Product tested by Health Canada and found to contain tadalafil | Wiggles Adult Video 980 The Queensway Etobicoke, ON |
Seized from the retail location | 2022-08-31 |
 |
USA V9 Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Wiggles Adult Video 980 The Queensway Etobicoke, ON |
Seized from the retail location | 2022-08-31 |
 |
Pillkings Plus 99K (liquid format) Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Boutique érotique Le Prince 5355 Mnt Saint-Hubert Saint-Hubert, QC |
Seized from the retail location | 2022-08-15 |
 |
Pillqueen Plus 99K (liquid format) Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Boutique érotique Le Prince 5355 Mnt Saint-Hubert Saint-Hubert, QC |
Seized from the retail location | 2022-08-15 |
 |
3800 Hard Rock Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
7k Sexual enhancement |
Labelled to contain yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Alien 2 Power Platinum 11000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Alien Power Platinum 11000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Black Panther #1 Sexual enhancement |
Labelled to contain yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Ginseing Red 2000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Jaguar 30000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Lucky Lady Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Master Zone 1500 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Platinum Rhino Love 20000 (red front packaging) Sexual enhancement |
Labelled to contain yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Platinum Rhino Love 20000 (black front packaging) Sexual enhancement |
Labelled to contain yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
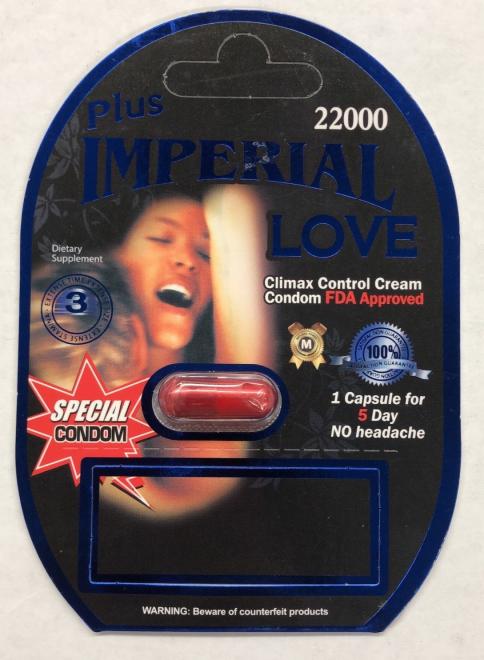
Plus Imperial Love 22000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Premier Love Platinum 22000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Premium Pro Power 3500 Sexual enhancement |
Labelled to contain yohimbe Product with similar packaging (previously seized) was tested and found to contain sildenafil |
Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Rhino 7 Platinum 5000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Rush Hour 72 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil, tadalafil, desmethyl carbodenafil and dithiodesmethyl carbodenafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Spanish Fly 20,000 (green capsule) Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Spanish Fly 22,000 (purple capsule) Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Stiff Rox Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
Super Panther 7K Sexual enhancement |
Labelled to contain yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
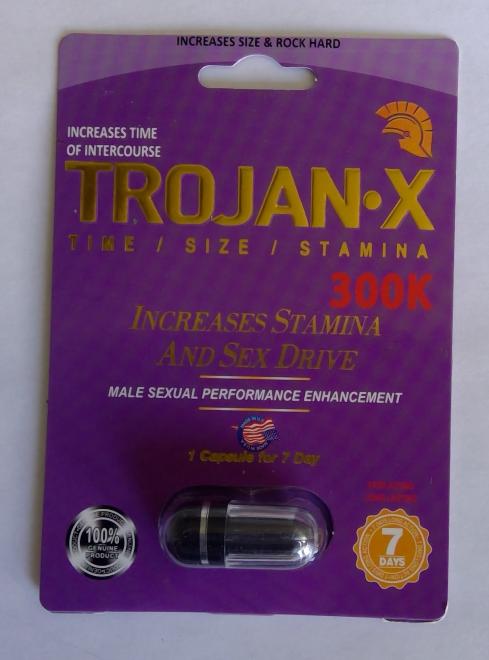
Trojan-X 300K Sexual enhancement |
Labelled to contain yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
XXL Ant 3000 Sexual enhancement |
Labelled to contain yohimbe | Grace Daily Mart 1579 Ellesmere Rd, Scarborough, ON |
Seized from the retail location | 2022-08-15 |
 |
MyoBlox Skywalk Peach Rings Workout supplement |
Product tested by Health Canada and found to contain levodopa | Amped Nutrition 314 Queen St S. Bolton, ON |
Seized from the retail location | 2022-07-26 |
 |
MyoBlox Skywalk Purple Haze Workout supplement |
Product tested by Health Canada and found to contain levodopa | Amped Nutrition 314 Queen St S. Bolton, ON |
Seized from the retail location | 2022-07-26 |
 |
MyoBlox Skywalk Red Wave Workout supplement |
Product tested by Health Canada and found to contain levodopa | Amped Nutrition 314 Queen St S. Bolton, ON |
Seized from the retail location | 2022-07-26 |
 |
Rhino 7 Platinum 5000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and yohimbe | Esso Gas Station 89 Lindsay Street S. Lindsay, ON |
Seized from the retail location | 2022-07-26 |
 |
Super Panther 500k Sexual enhancement |
Labelled to contain yohimbe | Esso Gas Station 89 Lindsay Street S. Lindsay, ON |
Seized from the retail location | 2022-07-26 |
 |
777K Sexual enhancement |
Labelled to contain yohimbe | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Plus Imperial Love 22000 Sexual enhancement |
Product tested by Health Canada and found to contain tadalafil and sildenafil | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Rhino 7 Platinum 5000 (large and small packaging) Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and yohimbe | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Spanish Fly 20,000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Stiff Rox Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Super Panther 7K Sexual enhancement |
Labelled to contain yohimbe | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Twin Rhino 69 Power 500K+ Sexual enhancement | Labelled to contain yohimbe | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
VIP Go Rhino Sexual enhancement | Labelled to contain yohimbe | Big Bee Convenience & Food Mart 800 Barton St E. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Platinum Rhino Love 2000 Sexual enhancement |
Labelled to contain yohimbe | Big Bee Convenience & Food Mart 155 Hunter St W. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Premier Love Platinum 22000 Sexual enhancement |
Product tested by Health Canada and found to contain tadalafil and sildenafil | Big Bee Convenience & Food Mart 155 Hunter St W. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
 |
Twin Rhino 69 Power 500K+ Sexual enhancement |
Labelled to contain yohimbe | Big Bee Convenience & Food Mart 155 Hunter St W. Hamilton, ON |
Seized from the retail location | 2022-07-12 |
| Infinity 10K Sexual enhancement |
Labelled to contain yohimbe | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Kangaroo Ultra 3000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Magnum Gold 24K Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
 |
Magnum Male Sexual enhancement XXL 1000K Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 |
| Magnum XXL 9800 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Passion Classic Sexual enhancement |
Labelled to contain yohimbe | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
 |
Pink Pussycat Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain hydroxythiohomosildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 |
| Platinum ZEN69 96000 Sexual enhancement |
Labelled to contain yohimbe | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Recharged Blue Rhino 79 Extreme 188K Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Rhino 8 Supreme 500K Sexual enhancement |
Labelled to contain yohimbe | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Sexy Lady Sexual enhancement |
Labelled to contain yohimbe Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
 |
Spanish Fly 22000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 |
| Swiss Navy Daily Supplement for Sexual Health Sexual enhancement |
Labelled to contain yohimbe | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Xcalibur Platinum 11000 Sexual enhancement |
Labelled to contain yohimbe | Love Secret Sex Shop 2279 Elgin Ave, Port Coquitlam, B.C. |
Seized from the retail location | 2022-07-12 | |
| Alien Power Platinum 11000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
| Black Panther #1 Sexual enhancement |
Labelled to contain yohimbe | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
| Black Panther #1 (3 pack) Sexual enhancement |
Labelled to contain yohimbe | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
| Black Panther Platinum 30K Sexual enhancement |
Labelled to contain yohimbe | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
| Lucky Lady Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
| ResErection! Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
| ResErection! (Bottle) Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
| Rhino Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain aminotadalafil | Love Den Romantic Accessories Ltd. Unit 121 – 735 Goldstream Avenue Langford, B.C. |
Seized from the retail location | 2022-07-12 | |
 |
777K Sexual enhancement |
Labelled to contain yohimbe | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Amsterdam Poppers |
Product with similar packaging (previously seized) was labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Blue Boy Original Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Double Scorpio Emerald Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
English Royale Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Gold Label Amsterdam Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Gold Rush Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Increasing Delay Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Jaguar 25000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Jaguar 30000 Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Japan Tengsu Sexual enhancement |
Labelled to contain sildenafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Jungle Juice Max Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Jungle Juice Platinum Poppers |
Product with similar packaging (previously seized) was tested and found to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Maca Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Master Zone 1500 (Blue) Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Passion Classic Sexual enhancement |
Labelled to contain yohimbe | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Pig Sweat Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Pink Pussycat Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain hydroxythiohomosildenafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Poseidon Platinum Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Power Panther Sexual enhancement |
Labelled to contain yohimbe | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Red Diamond Viagra Sexual enhancement |
Product was previously tested by Health Canada and found to contain sildenafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Red Mamba Sexual enhancement |
Labelled to contain yohimbe Product with similar packaging (previously seized) was tested and found to contain sildenafil and tadalafil |
Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Rising Phoenix 5K Sexual enhancement |
Labelled to contain yohimbe | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Rochefort Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Rush Original Poppers |
Labelled to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Rush Power Pak Poppers |
Product with similar packaging (previously seized) was tested and found to contain isobutyl nitrite | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Shiva Gold Sexual enhancement |
Product with similar packaging (previously seized) was tested and found to contain tadalafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Super Mamba Triple Maximum Sexual enhancement |
Labelled to contain yohimbe | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
USA Black Gold Sexual enhancement |
Labelled to contain sildenafil | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
X Panther 9X Sexual enhancement |
Labelled to contain yohimbe | Wiggles Adult Video 980 The Queensway, Etobicoke, ON |
Seized from the retail location | 2022-07-05 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream Skin lightening |
Labelled to contain tretinoin and 2% hydroquinone | Jedpar Health and Beauty Lethbridge, AB |
Seized from the warehouse location and removed from online sale | 2022-06-16 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Solution (Toner) Skin lightening |
Labelled to contain tretinoin and 2% hydroquinone | Jedpar Health and Beauty Lethbridge, AB |
Seized from the warehouse location and removed from online sale | 2022-06-16 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set Skin lightening |
Labelled to contain tretinoin and 2% hydroquinone | Jedpar Health and Beauty Lethbridge, AB |
Seized from the warehouse location and removed from online sale | 2022-06-16 |
 |
Aokeman Biaoge Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB Wah Loong Chinese Herbs Ltd. Wah Shun Chinese Herbs & Ginseng |
Seized from the retail location | 2022-06-16 |
 |
Chong Cao Lu Bian Wan Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB |
Seized from the retail location | 2022-06-16 |
 |
Chong Cao Lu Bian Wong Sexual enhancement |
Product tablet and capsule tested by Health Canada and capsule found to contain sildenafil | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB Wah Shun Chinese Herbs & Ginseng |
Seized from the retail location | 2022-06-16 |
 |
Chong Cao Shuang Shen |
Product tested by Health Canada and found to contain sildenafil and acetaminophen | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB Wah Loong Chinese Herbs Ltd. Wah Shun Chinese Herbs & Ginseng |
Seized from the retail location | 2022-06-16 |
 |
Germany Black Ants (Sheng Jing Pian) |
Product tested by Health Canada and found to contain sildenafil and ciprofloxacin | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB Wah Loong Chinese Herbs Ltd. Wah Shun Chinese Herbs & Ginseng |
Seized from the retail location | 2022-06-16 |
 |
Germany Black King Kong (De Guo Hei Jin Gang) Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB Wah Loong Chinese Herbs Ltd. |
Seized from the retail location | 2022-06-16 |
 |
King Kung Fu Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB |
Seized from the retail location | 2022-06-16 |
 |
Li Long Germany Black King Kong |
Product tested by Health Canada and found to contain sildenafil and ciprofloxacin | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB< Wah Loong Chinese Herbs Ltd. |
Seized from the retail location | 2022-06-16 |
 |
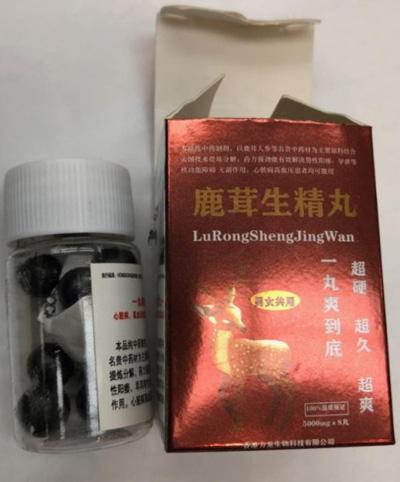
Lu Rong Sheng Jing Wan Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB Wah Loong Chinese Herbs Ltd. |
Seized from the retail location | 2022-06-16 |
 |
Lu Xie Shen Dan Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil, diclofenac and glyburide | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB |
Seized from the retail location | 2022-06-16 |
 |
Vigour 800 Sexual enhancement |
Product tested by Health Canada and found to contain sildenafil and ciprofloxacin | Wah Hing Chinese Ginseng Center 999 36 Street NE #800G Calgary, AB
Wah Shun Chinese Herbs & Ginseng |
Seized from the retail location | 2022-06-16 |
 |
Beneks' Fashion Fair Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Beneks' Hot Movate Gel |
Labelled to contain betamethasone dipropionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Clearbact Cream |
Labelled to contain betamethasone dipropionate and neomycin sulfate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Dermovate Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Diproson |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Esapharma Lemonvate |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Esapharma Movate |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Funbact-A Triple Action Cream |
Labelled to contain betamethasone dipropionate and neomycin sulfate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
H20 Jours Naturel Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
H20 Jours Naturel Cream Aloe Vera |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
H20 Jours Naturel Papaya Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
H20 Jours Naturel Pasteque Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Janet Papaya Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Kojic Clear Fast Action |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
L'abidjanaise Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
La Bamakoise |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Neoprosone-Gel Forte |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Visible Difference Cream |
Labelled to contain clobetasol propionate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
VixaSkineal Cream |
Labelled to contain clobetasol propionate, ketoconazole, and neomycin sulfate |
Akins International Foods |
Seized from the retail location |
2023-01-20 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set |
Labelled to contain hydroquinone and tretinoin |
More to Shoppe |
Removed from the warehouse location and removed from online sale |
2023-01-20 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Solution |
Labelled to contain hydroquinone and tretinoin |
More to Shoppe |
Removed from the warehouse location and removed from online sale |
2023-01-20 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream |
Labelled to contain hydroquinone and tretinoin |
More to Shoppe |
Removed from the warehouse location and removed from online sale |
2023-01-20 |
 |
DETO Fitness |
Product tested by Health Canada and found to contain sibutramine |
ZLS2 |
Removed from the warehouse location and from online sale |
2023-02-10 |
 |
Brilliant Skin Essentials Brilliant Rejuv Set |
Labelled to contain hydroquinone and tretinoin |
AR Pinoy |
Removed from the warehouse location and from online sale |
2023-02-10 |
 |
Brilliant Skin Essentials Brilliant Rejuv Topical Cream |
Labelled to contain hydroquinone and tretinoin |
AR Pinoy |
Removed from the warehouse location and from online sale |
2023-02-10 |
 |
Brilliant Skin Essentials Topical Solution (Toner) |
Labelled to contain hydroquinone and tretinoin |
AR Pinoy |
Removed from the warehouse location and from online sale |
2023-02-10 |
 |
Brilliant Skin Essentials Rejuvenating Facial Toner |
Product with similar packaging was tested and found to contain tretinoin |
AR Pinoy |
Removed from the warehouse location and removed from online sale |
2023-02-10 |
 |
Jekonmo Herbal Mixture |
Product tested by Health Canada and found to contain sildenafil |
Akins International Foods |
Seized from the retail location |
2023-02-10 |
 |
Blue Rhino Male Enhancement |
Product tested by Health Canada and found to contain sildenafil |
Cupid Boutique |
Seized from the retail location |
2023-02-10 |
 |
Just for Women |
Product tested by Health Canada and found to contain sildenafil |
Cupid Boutique |
Seized from the retail location |
2023-02-10 |
 |
Strong-SX Super Sex Pill |
Product tested by Health Canada and found to contain sildenafil |
Cupid Boutique |
Seized from the retail location |
2023-02-10 |
Issue
Health Canada is advising about unauthorized health products that may pose serious health risks. The table is updated when Health Canada finds unauthorized health products that are promoted for sexual enhancement, weight loss, as a workout aid, as "popper" or for lightening skin or treating skin conditions (such as eczema or psoriasis). These products are labelled to contain or have been tested and found to contain dangerous ingredients. Links to previous tables with affected products are also available below.
Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality. Unauthorized health products can pose many health dangers, including:
- They may contain ingredients not listed on the label. This includes ingredients like prescription drugs, possibly at doses exceeding maximum recommended amounts. Prescription drugs should be taken only under the supervision of a health care professional because they may cause serious side effects. Using a product that contains ingredients that the consumer is not aware of increases the chance of dangerous allergies and interactions with other medications and foods.
- The label may indicate a dangerous ingredient or combination of ingredients. For example, it could list a drug that should be available only by prescription from a health care professional, or a combination of ingredients that Health Canada does not permit because of serious health risks.
Health Canada maintains this page so that people can easily identify products they may have purchased and take appropriate action. You are encouraged to check back regularly for updates. Advisories on safety issues involving other types of products are available in the recalls and safety alerts database.
What you should do
- Stop using the products listed in the table. Consult your health care professional if you have used these products and have health concerns, and for advice on which health products are best for you and your family.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check if products have been authorized for sale by searching Health Canada’s Drug Product Database and Licensed Natural Health Product Database.
- Report any health product adverse events or complaints to Health Canada.
- See these helpful links for additional information on buying health products safely:
What companies should know:
- Selling unauthorized health products in Canada is illegal.
- When Health Canada identifies unauthorized products that may pose serious health risks, Health Canada takes appropriate action to prevent further distribution and informs Canadians. This includes working with the Canada Border Services Agency to help prevent further importation of unauthorized products.
Additional information
Previous recalls or alerts
Background
Acetaminophen is a drug that can pose serious health risks, including liver damage, if too much is taken. This may lead to liver failure or, in the most severe cases, death. Early signs of taking too much acetaminophen include nausea, vomiting, lethargy, sweating, loss of appetite and pain in the upper part of the abdomen or stomach. Abdominal pain may be the first sign of liver damage and may not be apparent for 24 to 48 hours. Acetaminophen may also cause serious skin reactions. Symptoms include blistering, skin reddening and rash.
Betamethasone dipropionate and clobetasol propionate are highly potent corticosteroid prescription drugs that are applied to the skin to treat inflammatory skin conditions. Side effects include skin irritation and, with prolonged use, skin weakening or deterioration. Adverse effects from using too much include decreased ability to fight infection, symptoms of adrenal gland suppression (i.e., low blood pressure, low blood sugar, weight loss, muscle pain, gastrointestinal problems and severe fatigue) or Cushing's syndrome (i.e., high blood pressure, high blood sugar, weight gain, muscle weakness, bone loss and severe fatigue), depending on how much has been absorbed. These drugs should not be used by pregnant or nursing people.
Ciprofloxacin in oral format (taken by mouth) is a prescription antibacterial drug used to treat bacterial infections of the lungs and airways, urinary system, prostate, skin, soft tissues, bones, and joints. It can also be used to treat diarrhea and certain sexually transmitted diseases caused by bacterial infections. Side effects may include nausea, diarrhea, joint pain, itching, and poor kidney function. Rare but serious side effects include disabling and possibly long-lasting tendonitis (tendon inflammation), tendon rupture (including the Achilles Tendon), nerve damage, neurological problems (including seizures), life-threatening heart issues (including heart electrical problems), myasthenia gravis (muscle disease), liver injury, and serious allergic reactions, including death. It is important to use this drug as directed by a health care professional. Misuse or overuse could lead to bacteria growth that will not be killed by ciprofloxacin, and could lead to bacterial resistance (the drug may not work in the future).
Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) used to relieve pain and inflammation (for example, pain and swelling associated with certain types of arthritis). In oral format (taken by mouth), it is a prescription drug. Diclofenac increases the risk of serious heart-related events (such as heart attack and stroke), high blood pressure, stomach or intestinal bleeding or ulcers, severe kidney or liver problems, and bleeding or clotting problems. It should not be used by patients who have: severe heart problems; stomach or intestinal conditions; bleeding disorders; a history of asthma or allergic-type reactions after taking diclofenac, acetylsalicylic acid (ASA) or other NSAIDs; severe kidney or liver problems; or high potassium. It should also not be used by children less than 16 years old, or by people in the third trimester of pregnancy or who are breastfeeding. Use in the first and second trimester of pregnancy must be carefully weighed by a doctor and may require increased monitoring. Diclofenac risks may be higher for patients taking other medications, as well as elderly patients.
Glyburide is a prescription drug used to treat patients with type 2 diabetes. Side effects may include severely low blood sugar (which can be life-threatening), stomach or intestinal problems, and serious skin and hypersensitivity reactions. Glyburide should not be used by people who: are less than 18 years of age; have type 1 diabetes; are pregnant or breastfeeding; or have liver disease or kidney impairment. It should also not be used during times of severe stress, such as during infection, mental stress, physical injury, or surgery. Risks may be higher for patients taking other medications, as well as elderly patients.
Hydroquinone is a prescription drug when it is greater than a 2% concentration and a natural health product at a concentration of 2% and under. It is used topically (applied to the skin) to lighten areas of darkened skin caused by different conditions (e.g., sun exposure, skin damage, pregnancy, medications or age). It should not be used by people who are allergic to hydroquinone or who are taking medicines that make their skin more sensitive to light. Hydroquinone is not recommended for pregnant or breastfeeding people, or for children. It should be used with caution in those who have previously had cancer. Side effects include skin reactions such as redness, dryness, cracked skin, burning, stinging, peeling, itching, increased sensitivity to sunlight, sunburn, blisters and scarring. It may cause skin discolouration (i.e., blue or black discolouration or white patches or spots) that, in some cases, can be disfiguring. In laboratory animals, it has been associated with cancer after long-term exposure.
Ketoconazole is a prescription antifungal drug typically used to treat skin and scalp infections. It should be used only under the supervision of a health care professional. When applied to skin, common side effects include severe irritation, itchiness and stinging. Contact dermatitis (an itchy or painful rash) and painful allergic reactions have been reported more rarely. Ketoconazole should not be used by pregnant or breastfeeding people unless directed by a health care professional.
Levodopa, also known as L-dopa, is a prescription drug that is combined with other drug ingredients in anti-Parkinson's medications. It should be used only under the supervision of a health care professional. Levodopa may interact with drugs prescribed for high blood pressure, and should not be used by breastfeeding or pregnant individuals. Among other contraindications or precautions, it should not be taken by people with narrow angle glaucoma, with untreated heart, liver, blood, kidney, lung (bronchial asthma) or hormonal diseases, with a history of melanoma or suspicious skin lesions, or those recently treated with monoamine oxidase (MAO) inhibitor drugs. Side effects requiring medical attention include: sudden onset of sleep; uncontrollable and abnormal movements of the face, eyelids, mouth, tongue, neck, arms, hands, or legs; severe or persistent nausea or vomiting; an irregular heartbeat or fluttering in the chest; hallucinations; feeling lightheaded when standing quickly; or unusual changes in mood or behaviour.
Lorcaserin was a prescription weight-loss drug that was never authorised for sale in Canada and was withdrawn from the United States market due to an increased occurrence of cancers among clinical trial participants. Other side effects include fatigue, headache, cough, sore throat, breathing difficulties, nausea, abdominal discomfort, back pain, irregular heartbeat, leg swelling, anxiety, mood change and depression. In diabetic patients, severe low blood sugar may occur.
Neomycin sulfate is an antibiotic prescription drug and should be used only under the supervision of a health care professional. Side effects include allergic reactions that range from mild skin reactions (itching, rash and hives) to severe, life-threatening allergic reactions (anaphylaxis). Side effects such as damage to nerve tissue or the central nervous system, inner ear, and organs responsible for hearing and balance, and reduced kidney function have occurred in patients taking neomycin orally (by mouth) or when applied on the skin to open wounds or damaged skin. As well, when not used as directed, neomycin sulphate could increase the risk of infections resistant to neomycin or other antibiotics. Neomycin sulphate should not be used by pregnant or breastfeeding people unless directed by a health care professional.
Prasterone is a controlled substance in Canada. It is approved as a prescription steroid hormone for vaginal insertion for the treatment of postmenopausal vulvovaginal atrophy, a medical condition characterized by the thinning and drying of the vaginal walls that may occur at menopause. It is illegal to sell controlled substances without proper authorization from Health Canada. Prasterone has not been approved in Canada for any other use, or in an oral (i.e., taken by mouth) format. Prasterone is transformed in the body into androgen and estrogen hormones. Androgens may increase the risk of liver damage, heart attack, stroke, reduced fertility, hardening of the arteries, the development of male characteristics in women (e.g., increased facial hair), and enlargement or tenderness of male breasts or nipples. Estrogens may increase the risk of high blood pressure, headache, breast and uterine cancer, and blood clots. People with a history of breast cancer, abnormal vaginal bleeding, or who are pregnant or breastfeeding should not use prasterone. Prasterone risks increase with the dose. The long-term health consequences of prasterone use are unknown.
"Poppers" is a slang term for products that contain alkyl nitrites. Despite being labelled for various uses such as leather cleaners, room odourizers or liquid incense, these products are inhaled or ingested by consumers for recreational purposes.
Alkyl nitrites, such as amyl nitrite, butyl nitrite and isobutyl nitrite are prescription drugs and should be used only under the supervision of a health care professional. Products containing alkyl nitrites may pose serious risks, including death, depending on the amount used, how frequently they are used and how long they are used for, as well as the person's health and the other medications they may be taking. Since it is difficult to control how much is inhaled, people can accidentally overdose. Swallowing these products can lead to serious medical complications and may be fatal. People with certain medical conditions (including recent head trauma, bleeding into the head, glaucoma, or heart disease) and those taking certain medications (particularly drugs used to treat erectile dysfunction, and other drugs such as high blood pressure medications, certain migraine drugs, and high doses of aspirin) or illicit drugs are at particular risk.
Sibutramine was previously used to treat obesity but is no longer authorized for sale in Canada because of its association with an increased risk of cardiovascular side effects such as heart attack and stroke. Other side effects include increased blood pressure and heart rate, dry mouth, difficulty sleeping and constipation.
Sildenafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g. nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Desmethyl carbodenafil and dithiodesmethyl carbodenafil, and Hydroxythiohomosildenafil are unauthorized substances that are similar to sildenafil and may pose similar health risks.
Tadalafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) since it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss. Nortadalafil is an unauthorized substance that is similar to tadalafil and may pose similar health risks.
Tretinoin in topical format (applied to the skin) is a prescription drug used to treat acne. Topical tretinoin should not be used during pregnancy as it has been associated with birth defects. It should also not be used by those who are breastfeeding or by children under 12 years old, or by individuals who have inflamed or irritated skin, have a previous skin cancer or undiagnosed skin lesions, who are taking medicines that make their skin more sensitive to light, or who have an allergy to tretinoin. Tretinoin may cause pain, irritation, itchiness, redness, or swelling at the site of application. It may damage skin, change skin colour, and increase sensitivity to sunlight or tanning beds, causing sunburns. Using tretinoin in combination with hydroquinone may increase some of the side effects of tretinoin.
Yohimbine is a prescription drug and should be used only under the supervision of a health care professional. Yohimbine is derived from yohimbe, a bark extract. The use of yohimbine or yohimbe may result in serious adverse reactions particularly in people with high blood pressure, or heart, kidney or liver disease. Side effects include increased blood pressure and heart rate, anxiety, dizziness, tremors, headache, nausea and sleep disorders. It should not be used by people who are pregnant or breastfeeding.
Details
Media and public enquiries
Media enquiries
Health Canada
(613) 957-2983
media@hc-sc.gc.ca
Public enquiries
(613) 957-2991
1-866 225-0709
info@hc-sc.gc.ca
Get notified
Receive notifications for new and updated recalls and alerts by category.