This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Risperdal Consta (risperidone powder for injectable prolonged-release suspension) – Needle Detachments Associated with the Needle Assembly Used for Gluteal Injection
- Starting date:
- April 7, 2010
- Posting date:
- May 25, 2010
- Type of communication:
- Dear Healthcare Professional Letter
- Subcategory:
- Drugs
- Source of recall:
- Health Canada
- Issue:
- Medical Devices, Product Safety
- Audience:
- Healthcare Professionals
- Identification number:
- RA-170002354
This is duplicated text of a letter from Janssen-Ortho Inc.
Contact the company for a copy of any references, attachments or enclosures.
Notice about Health Canada Advisories
April 7, 2010
Dear Health Care Professional,
Subject: Needle detachments associated with the needle assembly used for the gluteal injection of Risperdal® Consta® risperidone powder for Injectable Prolonged-Release Suspension
Janssen-Ortho, in cooperation with Health Canada, would like to provide you with important information concerning the needle assembly used for the gluteal injection for Risperdal® Consta® (risperidone).
Importantly, please note that the drug itself, the risperidone microspheres powder, along with the supplied diluent for reconstitution, are not affected by the needle assembly and detachment.
- Janssen-Ortho has received reports of detachments of the needle assembly for Risperdal® Consta®. Detachment can occur during assembly, before the injection is given, while injecting the patient, or during disposal of the syringe.
- Detachment could potentially lead to needle stick injury or incomplete drug injection.
- Risperdal® Consta® requires close attention to the step-by-step instructions for use to ensure successful administration. After following the Instructions for Use provided with product, Janssen-Ortho recommends that prior to injection, the health care professional recheck the connection between the orange Needle-Pro® safety device and the syringe to confirm that a secure attachment has been made.
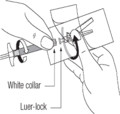
Attachment Site Illustration
There has been a recent increase in the number of reports of difficulty in attaching the orange Needle-Pro® safety device with the 2-inch gluteal injection needle onto the luer connection of the syringe included in the Risperdal® Consta® kit. In some cases, the orange Needle-Pro® safety device "turns back" after attachment to the syringe luer-lock (white collar).
Janssen-Ortho has received 83 complaints related to needle detachment over the past 3 months. This represents 1 complaint per 660 units sold. Of the 83, 5 reported missed or partial administration of a dose, which represents 1 case per 11,500 units sold. The analysis of the root cause of this issue is in progress. Manufacturing improvements have been identified and are progressing towards implementation as soon as possible.
Recommendations
Risperdal® Consta® requires close attention to the step-by-step instructions for use to ensure successful administration. After following the Instructions for Use provided with product, Janssen-Ortho recommends that prior to injection, the health care professional recheck the connection between the orange Needle-Pro® safety device and the syringe to confirm that a secure attachment has been made. Clinicians are advised to manage individual situations of missed or partial doses on a case-by-case basis.
Please report any occurrence of needle detachment immediately to Janssen-Ortho. In cases where needle detachment makes it unfeasible to administer the medication, Janssen-Ortho will replace the kit free of charge.
Risperdal® Consta® (risperidone) is indicated for the management of the manifestations of schizophrenia and related psychotic disorders. Risperdal® Consta® is also indicated as a monotherapy maintenance treatment in patients with bipolar I disorder, who have previously responded to oral antipsychotics or other anti-manic treatment, to delay occurrence of manic episodes.
Managing marketed health product-related adverse incidents depends on health care professionals and consumers reporting them. Reporting rates determined on the basis of spontaneously reported post-marketing adverse incidents are generally presumed to underestimate the risks associated with health product treatments. Any case of needle detachment, needle stick injury, incomplete injection of Risperdal® Consta®, or other adverse event, should be reported to Janssen-Ortho, or Health Canada (for serious/unexpected events), at the following addresses:
Janssen-Ortho Inc.
Medical Information Department
19 Green Belt Drive
Toronto, Ontario M3C 1L9
Telephone: 1-800-567-3331
Fax: 416-449-5248
MedinfoCanada@joica.jnj.com
Any suspected adverse reaction can also be reported to:
Canada Vigilance Program
Marketed Health Products Directorate
Health Canada
Address Locator: 0701E
Ottawa, Ontario, K1A 0K9
Telephone: 613-957-0337 or Fax: 613-957-0335
CanadaVigilance@hc-sc.gc.ca
To report an Adverse Reaction, consumers and health professionals may call toll free:
Telephone: 1-866-234-2345
Fax: 1-866-678-6789
Postage paid labels, the Canada Vigilance Reporting Forms and the Adverse Reaction Reporting Guidelines can be found on the MedEffect™ Canada Web site in the Adverse Reaction Reporting section. The Reporting Form is also in the Canadian Compendium of Pharmaceuticals and Specialties.
For other health product inquiries related to this communication, please contact Health Canada at:
Marketed Health Products Directorate
E-mail: mhpd_dpsc@hc-sc.gc.ca
Telephone: 613-954-6522
Fax: 613-952-7738
To change your mailing address or fax number, contact the Market Authorization Holder (industry).
original signed by
Dr. Cathy Lau,
Vice President, Regulatory Affairs and Quality Management
Images
Select thumbnail to enlarge - opens in a new window