This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Precautionary recall of birth control product: Esme-28
- Starting date:
- September 5, 2013
- Posting date:
- September 5, 2013
- Type of communication:
- Advisory
- Subcategory:
- Drugs
- Source of recall:
- Health Canada
- Issue:
- Labelling and Packaging
- Audience:
- General Public
- Identification number:
- RA-35477
- Issue
- Products affected
- What you should do
- Who is affected
- Report health or safety concerns
- Related AWRs
- Media enquiries
- Public enquiries
- What Health Canada is doing
- Images
Issue
Esme-28 (DIN 02388146), an oral contraceptive (birth control product), is being voluntarily recalled from the Canadian market by Mylan Pharmaceuticals. This action is further to their recall of a different birth control product, Freya-28, which was recalled on August 27, 2013, after one package was found to contain a placebo pill in place of an active pill.
Health Canada has been advised by Mylan Pharmaceuticals that they have been unable to rule out the possibility that Esme-28 was impacted by the same packaging issue involving Freya-28. Therefore, as a precaution, Mylan Pharmaceuticals is expanding their recall to include Esme-28. To date, no packaging errors have been identified with Esme-28.
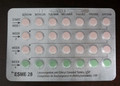
Packages of Esme-28 should have three rows of active (pink) pills and one row of placebo (green) pills.
Missing one or more active (pink) pills could result in reduced effectiveness for contraception, with the possibility of an unplanned pregnancy. As of August 24, 2013, no adverse reactions have been reported to Health Canada related to this issue.
Products affected
Esme-28 (Lot # F5042001C)
The company has informed Health Canada that Esme-21 is not impacted. Packages of Esme-21 should only have 21 active (pink) pills.
What you should do
- Consult your healthcare practitioner with any questions or concerns regarding the use of oral contraceptives, especially Esme-28.
- Use a non-hormonal method of birth control (such as condoms, spermicidal foam or gel) until another oral contraceptive can be obtained.
- Return unopened packages to your pharmacist.
- Report any adverse reactions potentially related to Esme-28 to Health Canada.
- Contact Health Canada's toll-free line at 1-800-267-9675 with questions or complaints about this product.
Who is affected
Canadians who have purchased or used Esme-28.
Report health or safety concerns
- Call toll-free at 1-866-234-2345
- Visit MedEffect Canada's web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
Related AWRs
Media enquiries
Health Canada
(613) 957-2983
Public enquiries
(613) 957-2991
1-866 225-0709
What Health Canada is doing
Health Canada will continue to monitor the situation and update Canadians and health care practitioners should new information or products be identified.
Images
Select thumbnail to enlarge - opens in a new window