This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
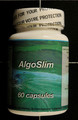
Information Update - Health Canada advises consumers not to use the unauthorized weight loss product, AlgoSlim, distributed via mail order by E Sélection
- Starting date:
- August 3, 2016
- Type of communication:
- Information Update
- Subcategory:
- Natural health products, Affects children, pregnant or breast feeding women
- Source of recall:
- Health Canada
- Issue:
- Product Safety, Product label update
- Audience:
- General Public
- Identification number:
- RA-59674
Issue
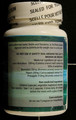
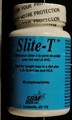
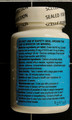
Health Canada is advising consumers not to use the unauthorized weight loss product AlgoSlim, distributed via mail order by E Sélection. The package does not contain unauthorized AlgoSlim and instead contains an authorized product, Slite-T, from a lot that expired in June 2012.
The product labelled as AlgoSlim contains ingredients not listed on the label (Camellia sinensis leaf and Garcinia cambogia). Consumers should also be aware that the following cautions and warnings for the authorised Slite-T product are hidden by the unauthorized AlgoSlim product labelling:
"Consult a health care practitioner before using the product if you are pregnant or breastfeeding. Consult a health care practitioner before using if you have an iron deficiency or if you have liver problems or if you show symptoms of liver problems."
Health Canada is following up and will inform Canadians should any new safety information arise.
| Product | NPN | Lot number | Expiry date |
|---|---|---|---|
| Slite-T labelled as AlgoSlim | 80015137 | UE1647 | June 2012 |
Report health or safety concerns
- Consult with your health care practitioner if you have used this product and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized products have an eight-digit DIN, DIN-HM or NPN.
- Verify if products have been authorized for sale by searching the Drug Product Database and the Licensed Natural Health Product Database.
- Report any adverse events to Health Canada.
- Report complaints about health products to Health Canada by calling toll-free 1-800-267-9675, or by completing an online complaint form.
Stay connected with Health Canada and receive the latest advisories and product recalls using social media tools.
Media enquiries
Health Canada
613-957-2983
Public enquiries
613-957-2991
1-866-225-0709
Images
Select thumbnail to enlarge - opens in a new window