Importation of US-authorized Epinephrine Injection USP, 1 mg/10 mL Single-Dose Pre-Filled Syringes Due to the Current Shortage of Canadian-Authorized Epinephrine Injection USP, 1 mg/10 mL Single-Use Syringes
Summary
See Key Messages below
Affected products
| Product Brand Name | Dosage Form, Strength, and Packaging Format | Country of Authorization and Identifying Code | Authorization Holder/ Manufacturer | Importer in Canada |
|---|---|---|---|---|
| Epinephrine Injection USP | Solution for injection 1 mg/10 mL (0.1 mg/mL) single-dose Luer-Jet Luer-Lock pre-filled syringe For intravenous use only. | United States of America NDC: 76329-3318-1 Stock NO. 3318 | Amphastar Pharmaceuticals, Inc. (USA)/ International Medication Systems, Limited | Amphastar Pharmaceuticals, Inc. (Canada) |
Issue
Given the shortage of Epinephrine Injection USP, 1 mg/10 mL single-use syringes in Canada, and to maintain continuity of supply, Health Canada has authorized the exceptional, temporary importation and sale of US-authorized Epinephrine Injection USP, 1 mg/10 mL single-dose pre-filled syringes with English-only labels by Amphastar Pharmaceuticals, Inc.
Audience
Healthcare professionals including pharmacists, cardiologists, anesthesiologists, critical care physicians and nurses, and emergency physicians and nurses.
Key messages
- Due to a shortage of Epinephrine Injection USP, 1 mg/10 mL single-use syringes in Canada, and given the medical importance of this drug, Health Canada has permitted the exceptional, temporary importation and sale of US-authorized Epinephrine Injection USP, 1 mg/10 mL single-dose pre-filled syringes with English-only labels by Amphastar Pharmaceuticals, Inc.
- Healthcare professionals are advised to:
- Be aware that the US-authorized product is labelled for intravenous use only. Unlike the Canadian authorized product by Pfizer Canada ULC, which is labelled for both intravenous and intracardiac use, the US-authorized product lacks a needle and is not intended for intracardiac administration.
- Be aware that the US-authorized product uses a Luer-JetTM syringe that is compatible with Luer-Lock intravenous tubing.
- Be aware that there are differences in the non-medicinal ingredients between the US-authorized product and Pfizer’s Canadian-authorized product, although they are not expected to be clinically meaningful.
- Refer to the outer carton of the US-authorized product for syringe assembly instructions (see Appendix 1), and refer to the Canadian Product Monograph for Epinephrine Injection USP, 1 mg/10 mL single-use syringes by Pfizer Canada ULC (DIN 00710814), available in English and French on Health Canada’s Drug Product Database, for all other information on proper use.
Background
In Canada, epinephrine injection is used to relieve respiratory distress from bronchospasm, manage hypersensitivity reactions, prolong the action of local anesthetics, and to restore cardiac rhythm during cardiac arrest, attacks of transitory atrioventricular (A-V) heart block and syncopal seizures, among various other uses.
Information for healthcare professionals
This product should be administered by appropriately trained healthcare professionals, familiar with its actions, characteristics, and hazards.
The US-authorized product has the same active ingredient (epinephrine), concentration (0.1 mg/mL) and dosage form (solution) as Pfizer’s Canadian-authorized Epinephrine Injection USP, 1 mg/10 mL single-use syringes. However, there are differences in the route of administration and the delivery system between the products, which are important to note, particularly in emergency situations where epinephrine injections are commonly used. These risks may not be readily apparent due to the similar packaging colours of both the US and Canadian products. Proper selection of the intended product must be verified to avoid confusion and prevent errors.
Healthcare professionals are advised that:
- The US-authorized product is labelled for intravenous use only. Unlike the Canadian-authorized product by Pfizer Canada ULC, which is labelled for both intravenous and intracardiac use, the US-authorized product lacks a needle and is not intended for intracardiac administration.
- The US-authorized product uses a Luer-JetTM syringe, which is compatible with Luer-Lock intravenous tubing; whereas, Pfizer’s Canadian-authorized product makes use of an Abboject® syringe, which includes a needle and provides needle-free access.
- There are differences in the non-medicinal ingredients between the US-authorized product and Pfizer’s Canadian-authorized product, although they are not expected to be clinically meaningful.
- The outer carton of the US-authorized product should be consulted for syringe assembly directions (see Appendix 1), while the Canadian Product Monograph for Epinephrine Injection USP, 1 mg/10 mL single-use syringes by Pfizer Canada ULC (DIN 00710814), available in English and French on Health Canada’s Drug Product Database, should be consulted for all other information on proper use.
The US-authorized product labels (see Appendix 1) are in English-only and do not include French text.
Healthcare professionals can find additional information about the US-authorized product in:
- The most current US-authorized prescribing information, available in English-only, on the US Food and Drug Administration website: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=211363.
- The package insert enclosed with the US-authorized product (one insert per 10 carton package), which is in English-only. Full digital copies of the US package insert (English and French translation) can be accessed from the following link: https://amphastar.com/epinephrine-usp-2.html.
Additionally, the US-authorized product does not have a Drug Identification Number (DIN) or a barcode that scans in medication management systems in Canada. A facility-generated sticker is recommended to enable barcode scanning and allow proper identification of the product being dispensed and administered.
Action taken by Health Canada
To help mitigate the shortage of Epinephrine Injection USP, 1 mg/10 mL single-use syringes in Canada, Health Canada has permitted the exceptional, temporary importation and sale of US-authorized Epinephrine Injection USP, 1 mg/10 mL single-dose pre-filled syringes by Amphastar Pharmaceuticals, Inc. and added this product to the List of Drugs for Exceptional Importation and Sale.
Health Canada has worked with Amphastar Pharmaceuticals, Inc. to prepare this alert for epinephrine injection. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication update will be further distributed through the MedEffect™ e-Notice email notification system.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any case of serious or unexpected side effects in patients receiving epinephrine injection should be reported to Amphastar Pharmaceuticals, Inc. or Health Canada.
Amphastar Pharmaceuticals Inc.
2000 Ellesmere Rd, Unit 16
Scarborough, ON, M1H 2W4
Canada
Telephone: 1-800-423-4136 or 1-909-980-9484
Fax: 1-909-980-6422
To correct your mailing address or fax number, contact Amphastar Pharmaceuticals Inc.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Regulatory Operations and Enforcement Branch
E-mail: hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Original signed by
Tony Marrs, MPH, MBA
EVP of Clinical Operations
Appendix 1.
A. Image of US-authorized Epinephrine Injection USP
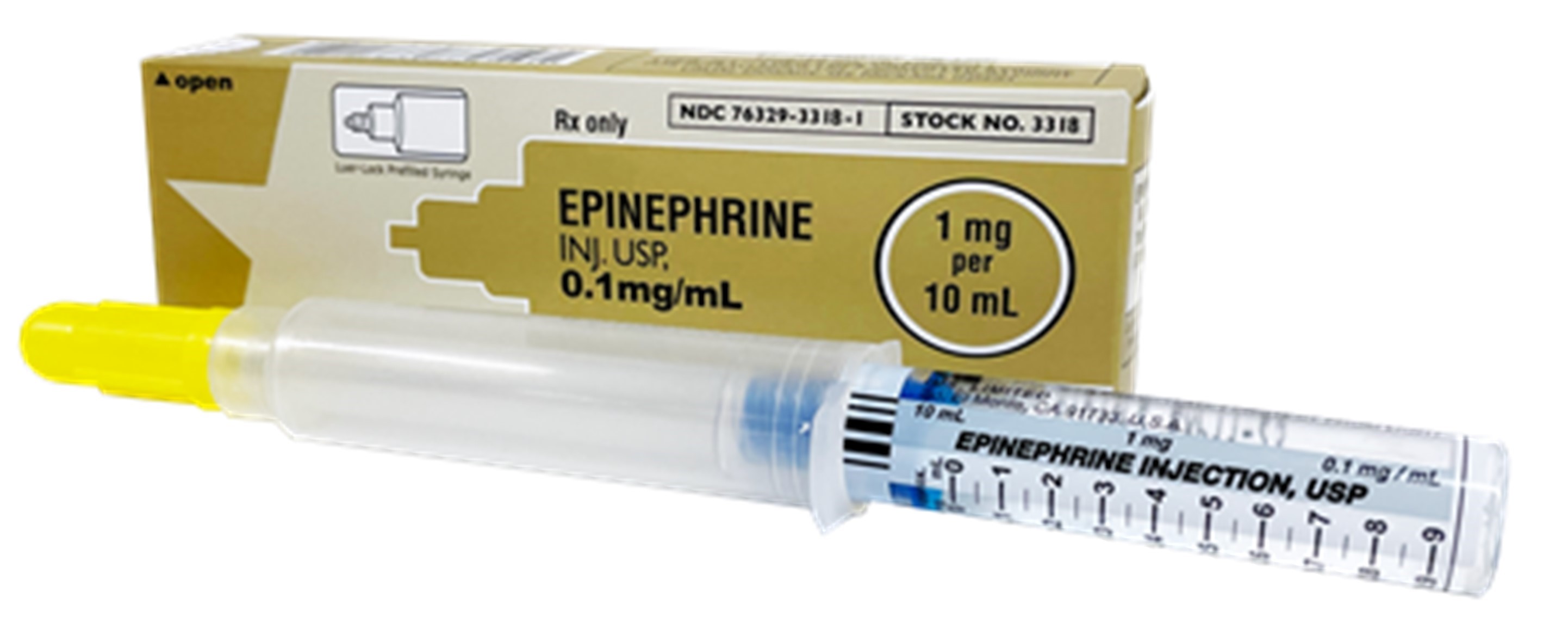
B. Image of US-authorized Epinephrine Injection USP, Inner Label
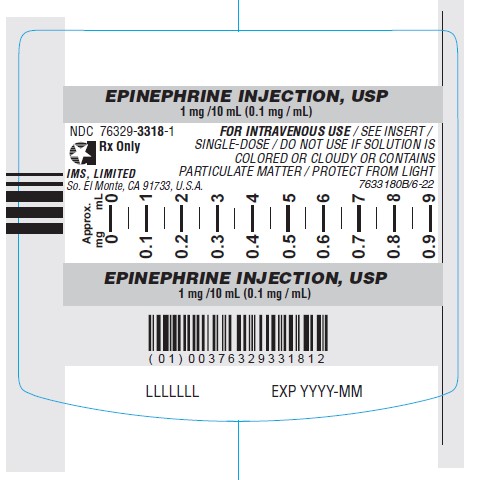
C. Image of US-authorized Epinephrine Injection USP, Outer (Carton) Label

Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.