Importation of UK-Authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion due to shortage of Canadian-authorized Potassium Chloride for Injection Concentrate
Summary
See Key Messages below
Affected products
| Product Name | Dosage Form, Strength, Packaging Format, and Route of Administration | Country of Origin and Identifying Code | Manufacturer | Importer and Supplier in Canada |
|---|---|---|---|---|
| Potassium Chloride 15% w/v Concentrate for Solution for Infusion | Concentrate for Solution for Infusion, 150 mg/mL potassium chloride, 10 mL plastic ampoule, Intravenous use | UK PL 24598/0003 | Noridem Enterprises Ltd., Cyprus | Juno Pharmaceuticals Corp. |
Issue
There is a shortage of Potassium Chloride for Injection Concentrate in Canada due to disruptions in the manufacturing of the products. Given the medical necessity of this product and to help mitigate the shortage, Health Canada has permitted the exceptional, temporary importation and sale of UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion with English-only labels.
Audience
Healthcare professionals including hospital pharmacists, critical care physicians, emergency physicians, and hospital physicians.
Key Message
- Due to a shortage of Potassium Chloride for Injection Concentrate (149 mg/mL) products in Canada and given the medical necessity of this product, Health Canada has permitted the exceptional, temporary importation and sale of UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion with English-only labels, and has added this product to the List of Drugs for Exceptional Importation and Sale.
- Healthcare professionals are advised that:
- The UK-authorized product has the same active ingredient, concentration, excipients, and dosage form as Canadian-authorized products. The UK-authorized and Canadian-authorized products are NOT for direct infusion and must be diluted prior to intravenous infusion.
- Despite the same concentration, the strength per total volume is expressed differently on the labels of the UK-authorized product (150 mg/mL) than on the labels of the Canadian-authorized products (149 mg/mL).
- In addition to differences in indications, there are significant differences in the packaging formats and related preparation instructions, and inner and outer labels, between Canadian-authorized products and the UK-authorized product (see the “Information for healthcare professionals” section).
Background Information
In Canada, Potassium Chloride for Injection Concentrate (149 mg / mL) is indicated for the treatment of potassium deficiency states when oral replacement therapy is not feasible, for the treatment of hypokalemic-hypochloremic alkalosis, and for the prevention and treatment of hypokalemia which may occur secondary to diuretic or corticosteroid administration. It may also be used in the treatment of cardiac arrhythmias due to digitalis intoxication.
There is a shortage of Potassium Chloride for Injection Concentrate in Canada due to disruptions in manufacturing of the products. Health Canada is permitting the temporary importation of UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion to help mitigate the current market shortage in Canada.
Information for healthcare professionals
The UK-authorized product is available in 10 mL plastic ampoules and has the same active ingredient, concentration, excipients, and dosage form as the Canadian-authorized products. The UK-authorized and Canadian-authorized products are NOT for direct infusion and must be diluted prior to intravenous infusion.
Despite the same concentration, the strength per total volume is expressed slightly differently on the labels of the UK-authorized product (150 mg/mL) than on the labels of the Canadian-authorized products (149 mg/mL).
In addition to differences in indications (see Table 1), there are key differences in the packaging formats and related preparation instructions, and inner and outer labels.
Table 1: Tabular summary of the Canadian-authorized Potassium Chloride for Injection Concentrate (149 mg/mL) products in shortage and UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion (150 mg/mL) exceptionally imported*
| Canadian-authorized Potassium Chloride for Injection Concentrate (149 mg/mL) products – currently in shortage | UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion |
|||
|
Pfizer Canada ULC DIN 00037869 |
Omega Laboratories Limited DIN 00402206DIN 02480034 |
B. Braun Medical Inc. DIN 02485699 |
|
|
| Authorized Indication(s) |
Treatment of potassium deficiency states when oral replacement therapy is not feasible.
|
Hypokalemic-hypochloremic alkalosis and for the prevention and treatment of hypokalemia which may occur secondary to diuretic or corticosteroid administration. It may be used in the treatment of cardiac arrhythmias due to digitalis intoxication.
|
Treatment of potassium deficiency states when oral replacement therapy is not feasible.
|
The prevention and treatment of potassium deficiency when oral replacement therapy is not feasible.
|
| Packaging Format(s)† |
10 mL and 20 mL single-use vials |
10 mL and 20 mL single-use vials 100 mL pharmacy bulk vial |
250 mL bag (pharmacy bulk package) | 10 mL plastic ampoule |
* The UK-authorized product is intended to support the shortage of comparable Canadian-authorized products highlighted in this table, regardless of differences in indication.
† Due to the differences in packaging formats between the Canadian-authorized and UK-authorized products, the preparation instructions differ between the products.
Healthcare professionals are advised that:
- There are significant differences between the inner and outer labels and packaging of the UK-authorized product (150 mg/mL) (see Appendix 1 for label images) and the Canadian-authorized products (149 mg/mL), which may increase the risk for medication errors such as:
- Packaging difference: The UK-authorized product being supplied as a plastic ampoule rather than as a vial or in plastic parenteral bags could increase the risk of inadvertent selection since the ampoule has a similar appearance to other products also available in 10 mL ampoules, e.g., sterile water for injection or sodium chloride injection 0.9% (see Table 1).
- Lack of critical warnings: All concentrated potassium products supplied in vials or ampoules in Canada must have distinctive label and package features such as black caps and black overseals bearing the words: “Must Be Diluted” in a contrasting colour, or black bands. The lack of distinctive display of those critical warnings on the UK-authorized product could increase the risk of inadvertent administration of the product by direct infusion without being diluted.
- Different expression of strength: The expression of strength in “mEq” is absent while the expression of strength in “mmol” is less prominently displayed on the labels of the UK-authorized product.
- Naming difference: The labels of the UK-authorized product display a prominent ‘K’. This single letter acronym is not standard practice to identify potassium chloride products in Canada.
- Concentrated potassium chloride has the potential for serious harm if administered in error. Proper selection of the intended drug product must be verified to prevent medication errors.
- The UK Summary of Product Characteristics (SmPC), which is available in English, should be consulted for instructions on preparation. The French-translated UK SmPC is available at junopharm.ca
- For the treatment of potassium deficiency states when oral replacement therapy is not feasible, the Canadian Product Monograph for the marketed product authorized for this indication should be used for information on the indications, contraindications, dosing, administration, storage and stability, warnings and precautions, adverse drug reactions, and drug interactions.
- For the treatment of hypokalemic-hypochloremic alkalosis, the prevention and treatment of hypokalemia which may occur secondary to diuretic or corticosteroid administration, and the treatment of cardiac arrhythmias due to digitalis intoxication, the Canadian Product Monograph for the marketed product authorized for these indications should be used for information on the indications, contraindications, dosing, administration, storage and stability, warnings and precautions, adverse drug reactions, and drug interactions.
Hospitals should also be aware that the UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion does not have a Drug Identification Number (DIN) or a barcode that scans in medication management systems in Canada. A facility-generated sticker could be helpful to enable barcode scanning and allow proper identification of the product being dispensed and administered.
Action taken by Health Canada
Given the medical necessity of Potassium Chloride for Injection Concentrate in Canada and to mitigate the shortage of this product, Health Canada has permitted the exceptional, temporary importation and distribution of UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion and has added this product to the List of Drugs for Exceptional Importation and Sale.
Health Canada has worked with Juno Pharmaceuticals Corp. to prepare this alert for Potassium Chloride 15% w/v Concentrate for Solution for Infusion. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication will be further distributed through the MedEffect™ e-Notice email notification system.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any case of serious or unexpected side effects in patients receiving Potassium Chloride 15% w/v Concentrate for Solution for Infusion should be reported to Juno Pharmaceuticals Corp. or Health Canada.
Juno Pharmaceuticals Corp.
2233 Argentia Road 402
Mississauga ON L5N2X7
1-855-819-0505
To correct your mailing address or fax number, contact Juno Pharmaceuticals Corp.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Regulatory Operations and Enforcement Branch
E-mail: hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Original signed by
Paul Varady
Appendix 1 - Images of UK-authorized Potassium Chloride 15% w/v Concentrate for Solution for Infusion with English-only labelling
Product Photos
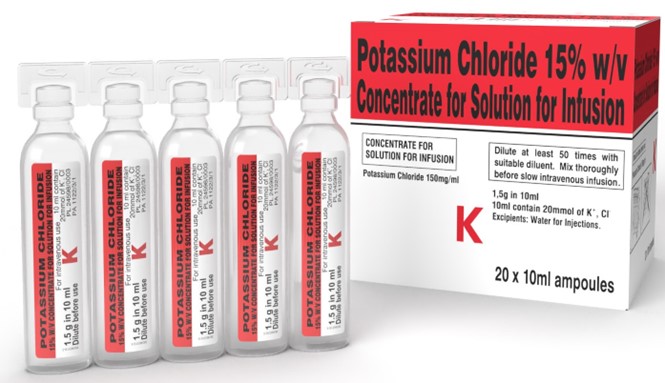
Vial label

POTASSIUM CHLORIDE
15% W/V CONCENTRATE FOR SOLUTION FOR INFUSION
For intravenous use
38246310-5
1.5 g in 10 ml.
Dilute before use
K
10mL contain 20mmol of K+, Cl-
PL 24598/0003
PA 1122/3/1
Carton Label
Carton Front panel

POTASSIUM CHLORIDE 15% w/v
CONCENTRATE FOR SOLUTION FOR INFUSION
CONCENTRATE FOR SOLUTION FOR INFUSION
Potassium Chloride 150mg/ml
Dilute at least 50 times with suitable diluent. Mix thoroughly before slow intravenous infusion.
1,5g in 10ml
10ml contain 20mmol of K+, Cl-
Excipients: Water for Injections.
K
20 x 10ml ampoules
Carton Top Panel

POTASSIUM CHLORIDE 15% w/v
CONCENTRATE FOR SOLUTION FOR INFUSION
Potassium Chloride
150mg/ml
5208063000233
20 x 10ml ampoules
K
Should be diluted before use
Back Carton Panel

POTASSIUM CHLORIDE 15% w/v
CONCENTRATE FOR SOLUTION FOR INFUSION
For Intravenous Use.
Use only if the solutions is clear and free from particles. Read the package leaflet before use. Use as directed by a medical practitioner. Discard after first use.
K
KEEP OUT OF THE SIGHT AND REACH OF CHILDREN
Marketing Authorisation Holder
noridem ENTERPRISES LIMITED
Evagorou & Makariou, Mitsi Building 3,
Office 115, 1065 Nicosia, Cyprus.
20 x 10 ml ampoules
Left Side Carton Panel

POTASSIUM CHLORIDE 15% w/v
CONCENTRATE FOR SOLUTION FOR INFUSION
Should be diluted before use
Potassium Chloride 150 mg/ml
After first opening: From a microbiological point of view, the product should be used immediately.
5208063000233
K
POM
PL 24598/0003
PA 1122/3/1
20 x 10ml ampoules
Right Side Carton Panel
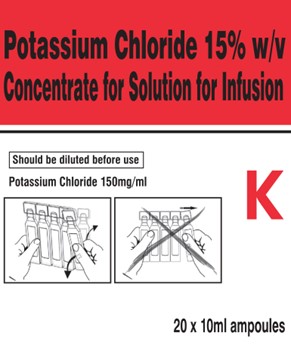
POTASSIUM CHLORIDE 15% w/v
CONCENTRATE FOR SOLUTION FOR INFUSION
Should be diluted before use
Potassium Chloride 150 mg/ml
K
20 x 10ml ampoules
Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.