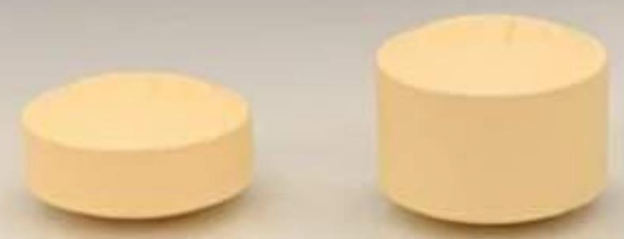
Donepezil 10 mg tablets: One lot recalled due to possible oversized tablets
Summary
If your pill bottle of Donepezil 10 mg tablets contains larger-than-normal tablets, or if you are unsure, return it to your pharmacy. Your pharmacist will check it and provide a replacement if needed. Contact a health care professional immediately if you or someone you are caring for are experiencing serious side effects.
Affected products
| Product | DIN | Lot number | Expiry date |
|---|---|---|---|
|
Donepezil (donepezil hydrochloride) 10 mg tablets |
02416425 |
645766 |
2024-11 |
Issue
Pro Doc Limitée is recalling one lot of Donepezil (donepezil hydrochloride) 10 mg tablets due to the possibility that some bottles might contain oversized tablets. Health Canada is warning patients and their caregivers that taking an oversized tablet could receive up to three times the intended dose (i.e. 30 mg), which may pose serious health risks.
Donepezil is a prescription drug used for the symptomatic treatment of mild, moderate, and severe Alzheimer’s disease.
A sudden increase in the dose of Donepezil, or exceeding the maximum recommended daily dose of 10 mg/day, may cause or worsen the most common adverse events such as nausea, diarrhea, vomiting, fatigue, loss of appetite, insomnia and muscle cramps. In general, clinical trials have found adverse events are more frequent with increasing age and in female patients. Cardiovascular adverse events such as slow heartbeat, heart block (a type of heart arrythmia) or loss of consciousness are more frequent in patients taking higher doses, and may also occur in patients with or without known underlying cardiovascular conditions. Immediate medical attention is needed for patients who experience symptoms of neuroleptic malignant syndrome while taking Donepezil, such as high fever, muscle stiffness, irregular blood pressure, pulse or heartbeats, confusion, agitation, or coma.
Higher than recommended doses Donepezil can result in cholinergic crisis, which is characterized by severe nausea, vomiting, salivation, sweating, slow heartbeats, low blood pressure, reduced breathing, loss of consciousness, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved.
Health Canada is monitoring the company’s recall, and its implementation of any necessary corrective and preventative actions to prevent the issue from reoccurring. The Department will inform the public if any new health risks are identified.
What you should do
- If your pill bottle of Donepezil 10 mg tablets contains larger-than-normal tablets, or if you are unsure, return it to your pharmacy. Your pharmacist will check it and provide a replacement if needed.
- Contact a health care professional immediately if you notice you or someone you are caring for are experiencing serious side effects.
- Contact Pro Doc Limitée by calling 1-800-361-8559 or emailing medinfo@prodoc.qc.ca
if you have questions about the recall.
- Report any health product-related side effects or complaints to Health Canada.
Additional information for health professionals:
- Health care professionals, such as pharmacists, should check bottles of Donepezil 10 mg tablets before dispensing and report any unusual packages to the company and to Health Canada.
Additional information
Details
Media and public enquiries
Media enquiries
Health Canada
(613) 957-2983
media@hc-sc.gc.ca
Public enquiries
(613) 957-2991
1-866 225-0709
Get notified
Receive emails about new and updated recall and safety alerts.