Distribution of NUVAXOVID with English-only Vial and Carton Labels
Summary
See Key Messages below
Affected products
Issue
NUVAXOVID was authorized by Health Canada on February 17, 2022. In order to provide earlier access to NUVAXOVID in the context of the global pandemic, Novavax, Inc. will distribute product vials, cartons and package inserts that are in English only for a period of time. The English-only vial and carton labels are missing some important Canadian-specific information normally found on Health Canada approved labels (see the Information for healthcare professionals section).
Audience
Healthcare professionals including infectious disease physicians, pharmacists, family physicians, public health officials, nurses and nurse practitioners.
Key Messages
- On February 17, 2022, NUVAXOVID™ (DIN 02525364) was authorized by Health Canada.
- NUVAXOVID is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older.
- In order to provide rapid access to NUVAXOVID, Novavax, Inc. will distribute product vials, cartons and package inserts that are in English only for a period of time (see Appendix A).
- Healthcare professionals are advised that:
- Important Canadian-specific information is absent from the vial and carton labels (see the Information for healthcare professionals section).
- The Canadian-specific labelling information, including the NUVAXOVID Canadian Product Monograph, can be accessed at www.NovavaxCovidVaccine.com, or by scanning the QR code on the English-only carton label or package insert. This information is also available on the federal government’s covid-vaccine.canada.ca website.
- The NUVAXOVID Canadian Product Monograph, in French and English, is also available on Health Canada’s Drug Product Database and should be referenced for complete product information.
Background information
NUVAXOVID is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older.
Given the public health emergency resulting from the current pandemic, Health Canada has authorized the importation, sale, and advertising of NUVAXOVID with vial and carton labels and package inserts that are in English only for the initial global distribution of the vaccine.
NUVAXOVID with global labels is the same as the Health Canada authorized NUVAXOVID in all aspects (i.e., formulation, strength, route of administration) and should be used in Canada for the same indication and per the same vaccination schedule. The Canadian Product Monograph for NUVAXOVID, which is approved by Health Canada and available in French and English, should be used for complete product information.
Information for healthcare professionals
Healthcare professionals are advised that:
- The approved Canadian Product Monograph, which is available in French and English on Health Canada’s Drug Product Database, the federal government’s covid-vaccine.canada.ca website or at www.NovavaxCovidVaccine.com, should be used for complete product information.
- The following important Canadian-specific information is absent from the vial and carton labels:
- Drug Identification Number (DIN)
- name and address of the Canadian DIN holder
- name and address of the Canadian importer and distributor
- all corresponding text in French
- In addition, the dosage form is indicated as “Dispersion for injection” rather than “Suspension for intramuscular injection”.
- The Canadian-specific information can be accessed at www.NovavaxCovidVaccine.com, or by scanning the QR code on the carton label or package insert. This information is also available on the federal government’s covid-vaccine.canada.ca website.
- For any medical information questions, contact Novavax Medical Information at 1-855-239-9172.
Action taken by Health Canada
Health Canada is permitting the use of a global English-only label for a limited period. Health Canada has imposed terms and conditions requiring Novavax, Inc. to provide vaccine supplies with Canadian-specific labels as soon as possible. Health Canada has made full labelling information available in French and English on the federal government’s covid-vaccine.canada.ca website.
Health Canada has worked with Novavax, Inc. to prepare this alert for NUVAXOVID. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians website. This communication will be further distributed through the MedEffect™ e-Notice email notification system, as well as through social media channels, including LinkedIn and Twitter.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any serious or unexpected side effects in patients receiving NUVAXOVID should be reported to your local Public Health Unit or Novavax, Inc.
Novavax, Inc.
21 Firstfield Road
Gaithersburg, MD, 20878
1-855-239-9172
To correct your mailing address or fax number, contact Novavax Inc. at 1-855-239-9172.
If a patient experiences a side effect following immunization, please complete the Adverse Events Following Immunization (AEFI) Form appropriate for your province/territory and send it to your local Health Unit.
For other health product inquiries related to this communication, contact Health Canada at:
Biologic and Radiopharmaceutical Drugs Directorate
E-mail: brdd.dgo.enquiries@hc-sc.gc.ca
Original signed by
Henrietta Ukwu, MD
Senior Vice President, Chief Regulatory Officer
Appendix A – Vial and carton labels for NUVAXOVID with English-only labelling
Vial
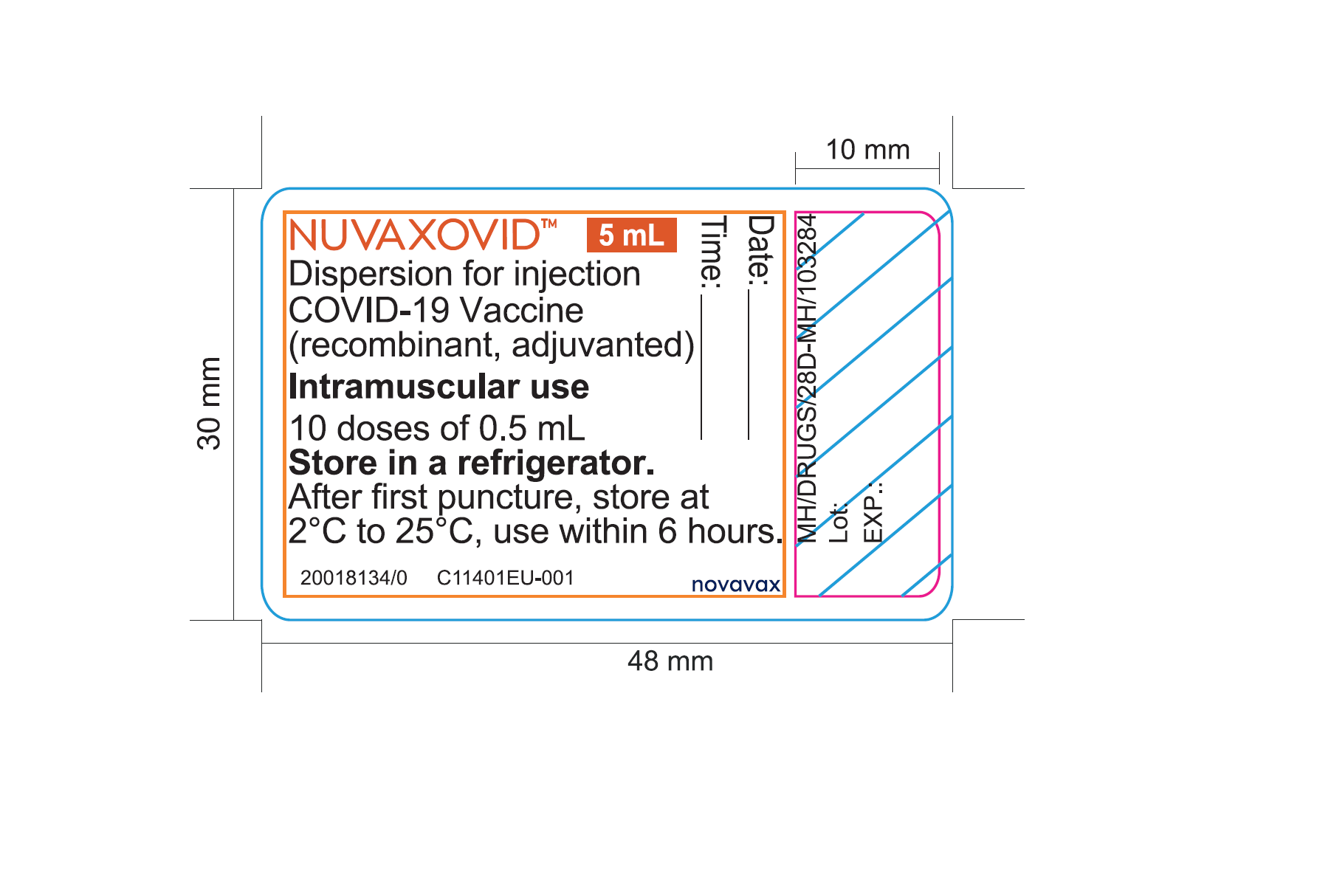
NUVAXOVID™ 5 mL
Dispersion for injection
COVID-19 Vaccine (recombinant, adjuvanted)
Intramuscular use
10 doses of 0.5 mL
Store in a refrigerator.
After first puncture, store at 2°C to 25°C, use within 6 hours.
Novavax
Date:
Time:
Lot:
EXP.:
Carton

Principle display panel:
NUVAXOVID™ 5 mL
Dispersion for injection
COVID-19 Vaccine (recombinant, adjuvanted)
Intramuscular use
10 multidose vials
Each vial contains 10 doses of 0.5 mL
Novavax
Back panel:
Each dose contains 5 micrograms of SARS-CoV-2 recombinant spike protein adjuvanted with Matrix- M.
Adjuvant Matrix-M: Fraction-A and Fraction-C of Quillaja saponaria Molina extract.
Excipients: disodium hydrogen phosphate heptahydrate, sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate dihydrate, sodium chloride, polysorbate 80, cholesterol, phosphatidylcholine (including all-rac-α-Tocopherol) potassium dihydrogen phosphate, potassium chloride, sodium hydroxide, hydrochloric acid, and water for injections.
Read the package leaflet before use.
Store in a refrigerator. Do not freeze.
Store in the original carton in order to protect from light.
After first puncture, store at 2°C to 25°C, use within 6 hours.
Side panel:
NUVAXOVID™ 5 mL
Dispersion for injection
COVID-19 Vaccine (recombinant, adjuvanted)
Intramuscular use
[QR code]
For more information, scan or visit
Side panel:
NUVAXOVID™ 5 mL
Dispersion for injection
COVID-19 Vaccine (recombinant, adjuvanted)
Intramuscular use
EU/1/21/1618/001
Novavax CZ a.s.
Bohumil 138, Jevany, 28163, Czechia
Top panel:
Lot:
EXP.:
barcode
Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.