Baxter Intravenous Solution Bags – Potential Leak During Spiking of Administration Port
Summary
See Key Messages below
Affected products
| Product Name | Package Size | Product Code | Market Authorization Holder | DIN | Lot Numbers/ Expiration Dates of products not recalled |
|---|---|---|---|---|---|
| 0.4% Lidocaine & 5% Dextrose Injection | 250 mL | JB0972 | Baxter Corporation | 00828602 | See Appendix A |
| 0.9% Sodium Chloride Injection, USP | 100 mL 250 mL | JB1302 JB1322 | Baxter Corporation | 00060208 | See Appendix A |
| Lactated Ringer’s Injection, USP | 250 mL | JB2322 | Baxter Corporation | 00061085 | See Appendix A |
| Metronidazole Injection, USP | 100 mL | JB3415 | Baxter Corporation | 00870420 | See Appendix A |
Issue
Intravenous bags of Baxter’s 0.4% Lidocaine & 5% Dextrose Injection 250 mL, 0.9% Sodium Chloride Injection, USP 100 mL and 250 mL, Lactated Ringer’s Injection, USP 250 mL, and Metronidazole Injection, USP 100mL from certain lots have the potential to leak during the process of spiking the administration port.
The affected lots identified in Appendix A are not being recalled at this time in order to prevent a shortage of these medically necessary products.
Audience
Healthcare professionals including physicians, nurses, pharmacists, and other medical and support personnel including inventory management and home healthcare agencies involved in the administration and handling of the following Baxter products:
- 0.4% LIDOCAINE & 5% DEXTROSE INJECTION (250ML)
- 0.9% SODIUM CHLORIDE INJECTION, USP (100ML, and 250ML)
- LACTATED RINGER’S INJECTION, USP (250ML)
- METRONIDAZOLE INJECTION, USP (100ML)
Key messages
- Certain lots of Baxter intravenous solution bags have the potential to leak during the process of spiking the administration port.
- Healthcare professionals are advised to:
- NOT use the product if the defect is observed.
- Follow guidance on the handling and verification of affected products (see the “Information for healthcare professionals” section) and
- Ensure preparedness at points of use where affected products are identified.
- Avoid using affected lots when feasible, particularly in situations requiring immediate product use (e.g., operating room, critical care and emergency). If only products from affected lots are available, additional measures should be considered, including the immediate supply of replacement bags at point of use and additional safety measures based on professional practice.
Background
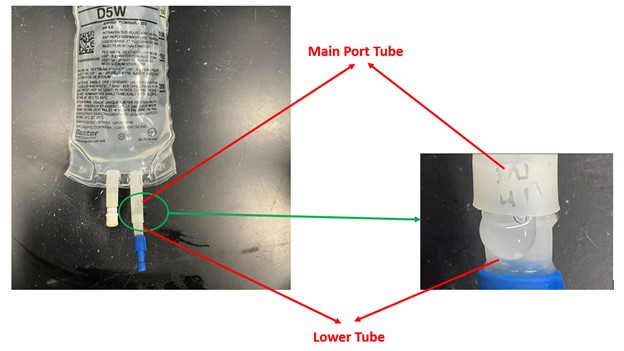
There have been reports of leaks and/or disconnections observed during spiking on the administration port at the connection of the lower tubing section and the main port tube (see Appendix B as an example).
Baxter identified the cause of the leakage of these intravenous bags as a combination of factors related to the manufacturing process. Baxter has implemented appropriate measures to address the leaks and prevent reoccurrence. Products from the affected lots were distributed by Baxter Corporation in Canada beginning April 28, 2023.
The safety risks that could occur because of a leaking solution bag include:
- Delay or interruption in therapy resulting in the administration of an inaccurate dose.
- Healthcare professionals or patients being exposed to the drug product, including hazardous medications (e.g., cytotoxic chemotherapeutic drugs) when these medications have been added to the bags.
- Patients receiving an infusion of contaminated solution due to microbial contamination of the solution at the point of use.
- Healthcare professionals or patients being exposed to harm due to the solution leaking onto electrical equipment.
Baxter is taking a phased-in recall approach to prevent a product shortage. In consultation with Health Canada, the affected lots identified in Appendix A are not being recalled at this time to ensure the continued availability of these medically necessary products. Further market actions may be taken in the future once sufficient non affected supply is available.
Baxter has previously initiated similar recalls of products affected by this issue, in consultation with Health Canada. For additional details, consult the Recalls and Safety Alerts Database on the Healthy Canadians Web Site.
Information for consumers (home patients)
Do NOT use the bags from affected lots if you see any damage or fluid leakage.
If you see any fluid leakage from the bags before or during treatment, stop using the product as there may be a potential risk of harm and consult your healthcare professional.
Gloves should be worn when handling the bag to avoid exposure to any cytotoxic drugs (such as some cancer drugs) that may have been added to the fluid in the bags.
Consult with your healthcare professional if you have used product from an affected lot, or are unsure and have questions or concerns about your health.
Information for healthcare professionals
- Visually inspect the container. If the administration port is damaged, detached, or not present, discard container, as solution sterility may be compromised.
- Check for administration port leaks after spiking the bag, by applying gentle pressure near the administration port. If leaks are found, discard solution as sterility may be compromised.
- Due to the possibility that a port leak may not be immediately observed, wait 1-2 minutes before adding medications.
- During the preparation of cytotoxic medications, follow pharmacy practice standards, and consider:
- Spiking the bag first with either the administration set or the CSTD (closed system transfer device) bag spike
- Inspect the bag prior to adding the medication
- Admix the medication after spiking the bag
- After adding the medication, leave the bag hanging under the hood for 1-2 extra minutes before sending to the units to ensure there is no leaking
- Regularly monitor for leakage from the bag during treatment and discontinue use if leakage is observed.
- Ensure spill kits are readily available on the unit when administering cytotoxic medications and staff are prepared, trained, and are aware of how to manage and handle hazardous materials
- Gloves should be worn when handling the bags to avoid exposure to any hazardous medications that may have been added to the bags
- Avoid using affected lots of Baxter IV solutions when feasible, particularly in situations requiring immediate product use (e.g., operating room, critical care and emergency). If only products from affected lots are available, additional measures should be considered, including the immediate supply of replacement bags at point of use and additional safety measures based on professional practice.
Action taken by Health Canada
Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication will be further distributed through the MedEffect™ e-Notice email notification system.
Health Canada has requested Baxter Corporation, the market authorization holder for 0.4% Lidocaine & 5% Dextrose Injection, 250 mL, 0.9% Sodium Chloride Injection, USP 100 mL and 250 mL, Lactated Ringer’s Injection, USP 250 mL, and Metronidazole Injection, USP 100mL to ensure that they inform all affected healthcare professionals, patients and personnel administering or handling these products.
Health Canada is monitoring the company’s implementation of any additional corrective and preventative actions. If additional safety information is identified, Health Canada will take appropriate action and inform Canadians as needed.
Report health or safety concerns
Health Canada’s ability to monitor the safety of marketed health products depends on healthcare professionals and consumers reporting adverse reactions and medical device incidents. Any serious or unexpected adverse reactions in patients receiving 0.4% Lidocaine & 5% Dextrose Injection, 250 mL, 0.9% Sodium Chloride Injection, USP 100 mL and 250 mL, Lactated Ringer’s Injection, USP 250 mL, and Metronidazole Injection, USP 100mL should be reported to Baxter Corporation or to Health Canada.
Baxter Corporation
7125 Mississauga Rd.
Mississauga, ON
L5N 0C2
To correct your mailing address or fax number, contact Baxter Corporation at fca_canada@baxter.com
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Health Product Compliance Directorate, Regulatory Operations and Enforcement Branch
E-mail: hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Original signed by
Brandon Gingrich
Senior Manager, Quality
Baxter Corporation – Canada
Appendix A: Affected Lots NOT Subject to Recall
| Product Code | Product Description | Lot Number | Expiration Date | DIN |
|---|---|---|---|---|
|
JB0972 (JB0972) |
0.4% LIDOCAINE & 5% DEXTROSE INJECTION (250ML) |
W3E11C1 | AUG 2024 | 00828602 |
|
JB2322 (JB2322P) |
LACTATED RINGER'S INJECTION, USP (250 ML) |
W3F22C1 | SEP 2024 | 00061085 |
| JB3415 | METRONIDAZOLE INJECTION, 500 mg / 100 mL, USP (100ML) |
W3E30C1 | NOV 2024 | 00870420 |
|
JB1302 (JB1302P) |
0.9% SODIUM CHLORIDE INJECTION, USP (100 ML) |
W3D25C0 |
APR 2024 |
00060208 |
| W3D26C0 | APR 2024 | |||
| W3D29C2 | APR 2024 | |||
| W3E03C0 | MAY 2024 | |||
| W3E04C0 | MAY 2024 | |||
| W3E09C0 | MAY 2024 | |||
| W3E18C0 | MAY 2024 | |||
| W3E30C0 | MAY 2024 | |||
| W3F06C0 | JUN 2024 | |||
| W3F13C0 | JUN 2024 | |||
| W3F21C0 | JUN 2024 | |||
| W3F28C0 | JUN 2024 | |||
| W3F29C0 | JUN 2024 | |||
| W3G11C0 | JUL 2024 | |||
| W3G12C0 | JUL 2024 | |||
| W3G13C0 | JUL 2024 | |||
| W3G17C3 | JUL 2024 | |||
| W3G18C0 | JUL 2024 | |||
|
JB1322 (JB1322P) |
0.9% SODIUM CHLORIDE INJECTION, USP (250 ML) |
W3D24B0 | JUL 2024 | 00060208 |
| W3D27C0 | JUL 2024 | |||
| W3E01B0 | AUG 2024 | |||
| W3E02B0 | AUG 2024 | |||
| W3E05C1 | AUG 2024 | |||
| W3E10B0 | AUG 2024 | |||
| W3E13C1 | AUG 2024 | |||
| W3E15B1 | AUG 2024 | |||
| W3E16B0 | AUG 2024 | |||
| W3E25C2 | AUG 2024 | |||
| W3F01C0 | SEP 2024 | |||
| W3F01C0S | SEP 2024 | |||
| W3F02C1 | SEP 2024 | |||
| W3F05B0 | SEP 2024 | |||
| W3F12B0 | SEP 2024 | |||
| W3F14C0 | SEP 2024 | |||
| W3F16B0 | SEP 2024 | |||
| W3F19B0 | SEP 2024 | |||
| W3F20B0 | SEP 2024 | |||
| W3F21B0KX | SEP 2024 | |||
| W3F21B0X | SEP 2024 | |||
| W3F22C0 | SEP 2024 | |||
| W3F30C0 | SEP 2024 | |||
| W3G10B0 | OCT 2024 | |||
| W3G11B0 | OCT 2024 | |||
| W3G12B0K | OCT 2024 | |||
| W3G12B0X | OCT 2024 | |||
| W3G14C0 | OCT 2024 | |||
| W3G15C0 | OCT 2024 | |||
| W3G17B0 | OCT 2024 | |||
| W3G17B0S | OCT 2024 | |||
| W3G18B0 | OCT 2024 | |||
| W3G18B0S | OCT 2024 |
Appendix B
The following photo is an example of where the leaks can occur for the implicated lots:
Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.