This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived – Important Drug Safety Information: EPREX (Epoetin Alfa) Sterile Solution: Revised Prescribing Information for Patients with Chronic Renal Failure
- Starting date:
- January 13, 2004
- Posting date:
- January 15, 2004
- Type of communication:
- Dear Healthcare Professional Letter
- Subcategory:
- Biologic/vaccine
- Source of recall:
- Health Canada
- Issue:
- New safety information
- Audience:
- Healthcare Professionals
- Identification number:
- RA-17000710
This is duplicated text of a letter from Janssen-Ortho Inc.
Contact the company for a copy of any references, attachments or enclosures.
Notice about Health Canada advisories
IMPORTANT DRUG SAFETY INFORMATION – EPREX* (epoetin alfa) Sterile Solution Revised Prescribing Information for Patients with Chronic Renal Failure
January 13, 2004
Dear Healthcare Professional:
Janssen-Ortho Inc., in consultation with Health Canada, has revised the following sections of the Product Monograph for EPREX (epoetin alfa) for patients with chronic renal failure (CRF): Warnings, Adverse Reactions, Dosage and Administration, and Information for the Patient. The Prescribing Information for the other registered indications remains unchanged.
In Canada, EPREX (epoetin alfa) is supplied in two formulations with different stabilizers, one containing polysorbate-80 (HSA-free PRE-FILLED SYRINGES), and the other containing Human Serum Albumin (HSA-containing MULTI-USE VIAL). For your convenience, revisions pertaining to the Dosage and Administration section of the Prescribing Information for patients with CRF are presented below:
Subcutaneous administration of recombinant human proteins may increase the risk of immunogenicity.Footnote 1
EPREX (HSA-Containing) Multi-Use Vial Formulation:
Where intravenous access is available (e.g. patients on hemodialysis), EPREX HSA-containing formulation should be administered intravenously.
Where intravenous access is not available (e.g. patients with renal insufficiency not yet undergoing dialysis or peritoneal dialysis patients), EPREX HSA-containing formulation may be administered subcutaneously following a risk/benefit assessment of this route of administration prior to initiating therapy.
EPREX Polysorbate-80 Containing (HSA-Free) PRE-FILLED SYRINGE Formulation:
EPREX (epoetin alfa) polysorbate-80 containing (HSA-free) formulation should be administered by the intravenous route only.
Please refer to the attached highlighted copy of the Prescribing Information for other important related revisions to the Warnings and Adverse Reactions sections. Please insert this revised Prescribing Information in your copy of the Compendium of Pharmaceuticals and Specialties (CPS) and use when prescribing or dispensing EPREX (epoetin alfa). The revised Product Monograph is also available on the Janssen-Ortho website at http://www.janssen-ortho.com.
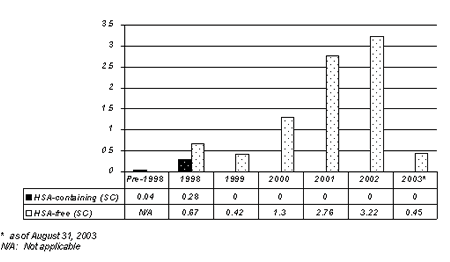
Janssen-Ortho Inc. informed you in November 2001 and in June 2002 of rare post-marketing reports of antibody-positive pure red cell aplasia (PRCA) in patients with CRF after months to years of treatment with EPREX (epoetin alfa) or other erythropoiesis regulating hormones. The large majority of these reports have been in patients with CRF treated with EPREX polysorbate-80 containing (HSA-free) pre-filled syringe formulation administered subcutaneously (SC). There is no evidence of an increased risk of antibody-positive PRCA associated with the polysorbate-80 containing (HSA-free) pre-filled syringe formulation administered IV.
There is also no evidence of an increased risk of antibody-positive PRCA associated with the HSA-containing multi-use vial formulation marketed only in Canada, whether administered IV or SC.
The reporting rate of antibody-positive PRCA has decreased significantly in 2003, with 5 reports worldwide as of August 31, 2003. Antibody-positive PRCA cases reported during the time period January 1, 1990 to August 31, 2003, stratified by formulation received, are presented in Figure 1.
Figure 1: Global Reports of Antibody Positive PRCA cases per 10,000 patient years of exposure by formulation received and year of Loss and Effect.
Janssen-Ortho Inc. will continue to keep you updated on an ongoing basis of any new scientific or medical information on PRCA as it becomes available. Further, additional information on pure red cell aplasia with erythropoietin products will be forthcoming from Health Canada.
The identification, characterization and management of drug-related adverse events are dependent on the active participation of healthcare professionals in adverse drug reaction reporting programs. Health care professionals are asked to report any suspected cases of PRCA or any other suspected adverse events in patients receiving EPREX (epoetin alfa) to Janssen-Ortho Inc. at the following address:
Janssen-Ortho Inc.
Drug Safety and Surveillance
19 Green Belt Drive
Toronto, ON M3C 1L9 or call toll free at 1-800-567-3331
email to dsscan@joica.jnj.com
Your professional commitment in this regard has an important role in protecting the well being of your patients by contributing to early signal detection and informed drug use.
Should you have any questions or require additional information regarding the use of EPREX (epoetin alfa), please contact Janssen-Ortho Inc. Medical Information Department at 1-800-567-3331 from 9:00 a.m. to 5:00 p.m. Monday to Friday Eastern Standard Time.
Sincerely
original signed by
Wendy Arnott, Pharm. D.
Vice-President
Regulatory, Quality and Safety
Any suspected adverse incident can also be reported to:
Canadian Adverse Drug Reaction Monitoring Program (CADRMP)
Marketed Health Products Directorate
HEALTH CANADA
Address Locator: 0701C
OTTAWA, Ontario, K1A 0K9
Tel: 613-957-0337 or Fax: 613-957-0335
Toll free for consumers and health professionals:
Tel: 866-234-2345, Fax: 866-678-6789
cadrmp@hc-sc.gc.ca
The AR Reporting Form and the AR Guidelines can be found on the TPD web site or in The Canadian Compendium of Pharmaceuticals and Specialties.
* All trademark rights used under license.
Footnotes
- Footnote 1
-
Porter S. Human Immune Response to Recombinant Human Proteins. J Pharm Sci 2001;90:1-11.
Images
Select thumbnail to enlarge - opens in a new window