Weed Me Inc. recalls one lot of Diamond District Sativa Pre-Rolls Cannabis Extract
Summary
Verify if your product is affected.
Affected products
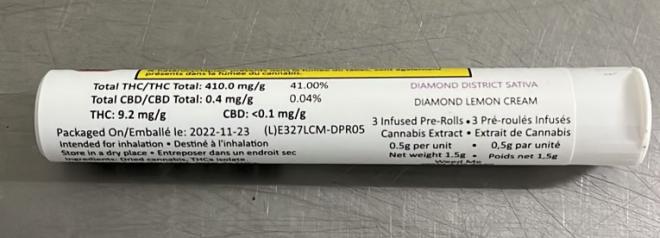
| Product name | Lot Number | Product Size | Packaging Date | Printed Values | Actual Values |
|---|---|---|---|---|---|
| Diamond District Sativa Pre-Rolls | E327LCM-DPR05 | 3 units x 0.5g/unit (1.5 g) | 2022-11-23 | THC: 9.2 mg/g Total THC: 410.0 mg/g | THC: 2.6 mg/g Total THC: 264.4 mg/g |
Issue
This recall involves one lot of Diamond District Sativa Pre-Rolls cannabis extract sold by Weed Me Inc. This product was sold through the Ontario Cannabis Store and through authorized retailers in Ontario.
Hazard identified
The product mistakenly contains only dried cannabis. The product label has incorrect cannabinoid values, where the THC and total THC labelled is higher than the actual THC and total THC.
To date, neither Weed Me Inc. nor Health Canada have received any complaints related to the recalled lot. Neither Weed Me Inc. nor Health Canada have received any adverse reaction reports for the recalled lot.
Number sold
1230 units of recalled product were sold.
Time period sold
The recalled product was sold from November 23 to December 2, 2022.
What you should do
Consumers should verify whether their product is affected. If you wish to return affected product, please contact the retail store where the product was purchased.
Health Canada would like to remind Canadians to report any health or safety complaints related to the use of this cannabis product or any other cannabis product by filling out the online complaint form.
Additional information
Details
Recalling Firm
Weed Me Inc.
Ontario
Canada
Tel: 1-866-410-4040
Distributor
Ontario Cannabis Store
Ontario
CANADA
Tel: 1-888-910-0627
Get notified
Receive emails about new and updated recall and safety alerts.