Unauthorized injectable drugs and medical devices seized from Vivian Spa in Mississauga, ON, may pose serious health risks
- Starting date:
- October 19, 2021
- Type of communication:
- Advisory
- Subcategory:
- Drugs, Medical Device
- Source of recall:
- Health Canada
- Issue:
- Unauthorized products
- Audience:
- General Public
- Identification number:
- RA-76677
Last updated: 2021-10-19
Summary
- Product: Several unauthorized injectable drugs and medical devices for cosmetic purposes, including products labelled to contain botulinum toxin type A or hyaluronic acid (a dermal filler).
- Issue: Health Canada seized these products from Vivian Spa in Mississauga, ON, because they are unauthorized and may pose serious health risks.
- What to do: Do not use these unauthorized products. Consult your healthcare professional if you have used or have been administered these products at Vivian Spa or any other location, and have health concerns. When receiving botulinum toxin or hyaluronic acid dermal filler treatments, ask your healthcare professional to show you the product label and verify that the health product has been authorized for sale by Health Canada.
Issue
Health Canada has seized several injectable drugs and medical devices, including products labelled to contain botulinum toxin type A or hyaluronic acid from Vivian Spa, located at 11-3415 Dixie Road, Mississauga, Ontario, because they are unauthorized and may pose serious health risks. These products may have been administered as treatments for cosmetic purposes.
Selling unauthorized health products in Canada is illegal. Unauthorized health products have not been approved by Health Canada, which means they have not been assessed for safety, effectiveness and quality and may pose serious health risks. They may lack active ingredients, or contain ingredients, additives or contaminated ingredients not listed on the label that could interact with other medications and foods. For all these reasons, unauthorized health products could cause serious health effects.
Botulinum toxin type A is used to treat severe muscle spasms in the neck, eye and foot, as well as chronic migraines, urinary incontinence, and excessive sweating. It is also used for cosmetic purposes to treat facial wrinkling. Authorized botulinum toxin type A products should only be used under specialist supervision and only if the benefits of treatment are considered to outweigh the risks. Potential risks associated with injecting an unauthorized Botulinum toxin type A product can range from mild local paralysis to death. All products administered by injection in Canada must be authorized for sale by Health Canada.
Unauthorized injectable drugs carry significant risk due to potential for infection, scarring, allergic reaction, and poor outcomes. These products have not been assessed for safety, effectiveness or quality.
Unauthorized medical devices that have not been licenced for sale in Canada may not meet Health Canada’s standards for safety, effectiveness or quality.
What you should do
- Do not use these unauthorized health products. Consult with a healthcare professional if you think you may have been administered these products at this spa and have health concerns.
- When receiving botulinum toxin or hyaluronic acid dermal filler treatments, ask your healthcare professional to show you the product label and verify that the health product has been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products are currently authorized for sale by searching Health Canada's Drug Product Database, Licensed Natural Health Products Database or Medical Devices Active Licence Listing.
- Report any health product adverse events or complaint to Health Canada.
Affected products
Unauthorized injectable drugs and Unauthorized medical devices
Product description
| Photo | Product | Risk |
|---|---|---|
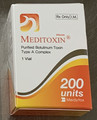
| Meditoxin, Purified Botulinum Toxin Type A | Unauthorized injectable drug (labelled to contain botulinum toxin type A) | |
| Toxta Inj. Botulinum Toxin Type A | Unauthorized injectable drug (labelled to contain botulinum toxin type A) | |
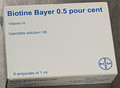
| Biotine Bayer 0.5% Vitamin H Injectable solution IM | Unauthorized injectable drug | |
| Mizain Voluma with Lidocaine | Unauthorized medical device | |
| Mizain Hyaluronic Acid 50cc | Unauthorized medical device | |
| Ammi Mulkwang Capture Time PDRN | Unauthorized medical device | |
| Royal Premium Family #5, Prosthesis Biomaterial with Lidocaine | Unauthorized medical device | |
| Juvéderm Voluma with Lidocaine | Unauthorized medical device | |
| Mole Removal Plasma Pen | Unauthorized medical device |
Media enquiries
Health Canada
(613) 957-2983
media@hc-sc.gc.ca
Public enquiries
(613) 957-2991
1-866 225-0709
info@hc-sc.gc.ca
Images
Select thumbnail to enlarge - opens in a new window