SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) COVID-19 Vaccine with English-only Vial and Carton Labels
Summary
See Key Messages below
Affected products
SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) (0.10 mg/mL), 2.5 mL multidose vial, dispersion for intramuscular injection, 5 doses of 0.5 mL each.
DIN: 02532352
Manufacturer: ModernaTX, Inc.
Canadian Importer and Distributor: Innomar Strategies
Issue
Health Canada authorized SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) on November 3, 2022. In order to provide rapid access to the vaccine, Moderna will distribute product vials and cartons labelled in English only with the brand name “SPIKEVAX Bivalent Original / Omicron BA.4/BA.5” for a period of time. Important Canadian-specific information is absent from these labels (see the Information for healthcare professionals section).
Both Moderna bivalent COVID-19 vaccine presentations, SPIKEVAX Bivalent Original / Omicron BA.4/5 and SPIKEVAX Bivalent (targeting Original / Omicron BA.1), have the same royal blue vial cap as monovalent SPIKEVAX 0.10 mg/mL, 2.5 mL. The royal blue cap represents the 0.10 mg/mL product concentration and should NOT be used alone to identify the product. To avoid medication errors, pay careful attention to the vaccine name and the vial and carton labels.
The vial and carton label of SPIKEVAX Bivalent Original / Omicron BA.4/BA.5 have a grey border and the concentration (0.1 mg/mL) is indicated in a grey band. The booster dose (50 mcg) and dose volume (0.5 mL) do not appear on the English-only vial and /or carton labels.
Audience
Healthcare professionals including infectious disease physicians, pharmacists, family physicians, public health officials, nurses and nurse practitioners, healthcare professionals at vaccination sites.
Key messages
- On November 3, 2022, Health Canada authorized Moderna’s SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) mRNA COVID-19 vaccine, DIN 02532352, which encodes for the Spike (S) protein of SARS-CoV-2 original variant and Omicron variant (B.1.1.529 [BA.4-BA.5]).
- SPIKEVAX Bivalent (Original / Omicron BA.4/5) vaccine is indicated as a booster dose for active immunization against coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 virus in individuals 18 years of age and older.
- In order to provide rapid access to SPIKEVAX Bivalent (Original / Omicron BA.4/5), Moderna will distribute product vials and cartons with English-only labels with the brand name “SPIKEVAX Bivalent Original / Omicron BA.4/BA.5” (see Appendix A), for a period of time.
- Existing supplies of SPIKEVAX Bivalent (targeting Original / Omicron BA.1) (elasomeran / imelasomeran), 0.10 mg/mL, 2.5 mL multidose vial and monovalent SPIKEVAX (elasomeran), 0.10 mg/mL, 2.5 mL multidose vial and 0.20 mg/mL, 5 mL multidose vial, continue to be available at this time.
- Healthcare professionals are advised that:
- Both Moderna bivalent COVID-19 vaccine presentations, SPIKEVAX Bivalent (Original / Omicron BA.4/5) and SPIKEVAX Bivalent (targeting Original / Omicron BA.1), have the same royal blue vial cap as monovalent SPIKEVAX 0.10 mg/mL, 2.5 mL multidose vial. The royal blue cap represents the 0.10 mg/mL product concentration and should NOT be used alone to identify the product. To avoid medication errors, pay careful attention to the vaccine name and the vial and carton labels.
- Important Canadian-specific information is absent from the vial and carton labels (see the Information for healthcare professionals section).
- The Canadian-specific labelling information including the SPIKEVAX Bivalent (Original / Omicron BA.4/5) Product Monograph and training materials can be accessed at modernacovid19global.com/ca, or by scanning the QR code on the English-only vial and carton labels. This information is also available on the federal government’s covid-vaccine.canada.ca website. The Canadian SPIKEVAX Bivalent (Original / Omicron BA.4/5) Product Monograph in English and French is also available on Health Canada’s Drug Product Database.
- Moderna will develop Canadian-specific vial and carton labels for SPIKEVAX Bivalent (Original / Omicron BA.4/5) in English and French, and make them available on the modernacovid19global.com/ca website at a future date.
Background
SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) mRNA vaccine is indicated as a booster dose for active immunization against coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in individuals 18 years of age and older.
Given the public health emergency resulting from the current pandemic, Health Canada has authorized the importation, sale, and advertising of SPIKEVAX Bivalent (Original / Omicron BA.4/5) with vial and carton labels that are in English only with the brand name “SPIKEVAX Bivalent Original / Omicron BA.4/BA.5” for the initial global distribution of the vaccine.
The Canadian Product Monograph for SPIKEVAX Bivalent (Original / Omicron BA.4/5), which is approved by Health Canada and available in English and French, should be used for complete product information.
Information for healthcare professionals
Healthcare professionals are advised that:
- On November 3, 2022, Health Canada authorized SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) COVID-19 vaccine.
- In order to provide rapid access to SPIKEVAX Bivalent (Original / Omicron BA.4/5), Moderna will distribute product vials and cartons labelled in English only with the brand name “SPIKEVAX Bivalent Original / Omicron BA.4/BA.5” for a period of time.
- SPIKEVAX Bivalent Original / Omicron BA.4/BA.5, 0.1 mg/mL, 2.5 mL has the same royal blue vial cap as SPIKEVAX Bivalent (targeting Original / Omicron BA.1), 0.10 mg/mL, 2.5 mL and monovalent SPIKEVAX 0.10 mg/mL, 2.5 mL.
- The royal blue cap represents the product concentration (0.10 mg/mL) and should NOT be used alone to identify the product.
- To avoid medication errors, pay careful attention to the SPIKEVAX vaccine name and the vial and carton labels.
- The vial and carton labels of SPIKEVAX Bivalent Original / Omicron BA.4/BA.5 have a grey border and the concentration (0.1 mg/mL) is in a grey band.
- The booster dose (50 mcg) and dose volume (0.5 mL) do not appear on the English-only vial and/or carton labels.
- The Canadian-specific information, including the SPIKEVAX Bivalent (Original / Omicron BA.4/5) Product Monograph and training materials, can be accessed at modernacovid19global.com/ca, or by scanning the QR code on carton and vial labels. This information is also available on the federal government’s covid-vaccine.canada.ca website. The Canadian SPIKEVAX Bivalent (Original / Omicron BA.4/5) Product Monograph in English and French, which is also available on Health Canada’s Drug Product Database, should be used for complete product information.
- The following important Canadian-specific information is absent from the vial and carton labels:
- Drug Identification Number (DIN)
- name and address of the Canadian DIN holder
- name and address of the Canadian importer and distributor
- all corresponding text in French
- Canadian brand name SPIKEVAX Bivalent (Original / Omicron BA.4/5)
Action taken by Health Canada
Health Canada is permitting the use of English-only labels with the brand name “SPIKEVAX Bivalent Original / Omicron BA.4/BA.5” for a limited period. Health Canada has imposed terms and conditions requiring Moderna to provide vaccine supplies with Canadian-specific labels as soon as possible. Health Canada has made full labelling information available in English and French on the federal government’s covid-vaccine.canada.ca website.
Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication update will be further distributed through the MedEffect™ e-Notice email notification system, as well as through social media channels, including LinkedIn and Twitter.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any serious or unexpected side effects in patients receiving SPIKEVAX Bivalent (Original / Omicron BA.4/5) should be reported to your local Health Unit or Moderna.
Moderna Biopharma Canada Corporation
c/o SE Corporate Services Ltd.,
Suite 1700, Park Place, 666 Burrard Street,
Vancouver, BC V6C 2X8
Telephone: 1-866-663-3762
Fax: 1-866-599-1342
To correct your mailing address or fax number, contact Moderna Biopharma Canada Corporation at 1-866-MODERNA (1-866-663-3762).
If a patient experiences a side effect following immunization, please complete the Adverse Events Following Immunization (AEFI) Form appropriate for your province/territory and send it to your local Health Unit.
For other health product inquiries related to this communication, contact Health Canada at:
Biologic and Radiopharmaceutical Drugs Directorate
E-mail: brdd.dgo.enquiries@hc-sc.gc.ca
Original signed by
Leslie Madden, BSc, MBA, LLM
Senior Director, Head of Regulatory Science & Quality Assurance, Canada
ModernaTX, Inc.
Appendix A – Vial and carton labels for SPIKEVAX Bivalent Original/Omicron BA.4/BA.5 (elasomeran/davesomeran) with English-only labelling*
Vial Label
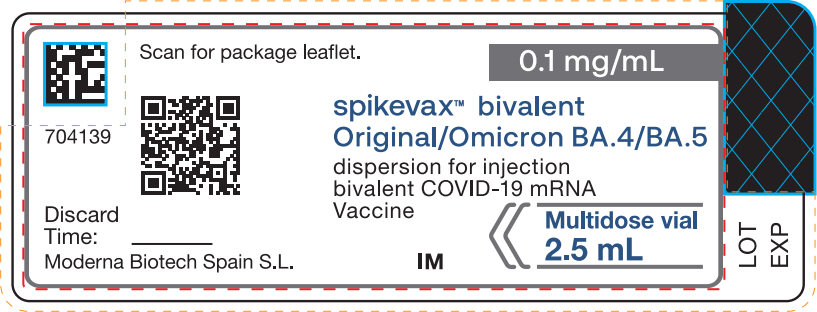
|
[2D matrix code*] 704139 Scan for package leaflet. [QR code]
Discard Time: ________________ Moderna Biotech Spain S.L.
|
0.1 mg/mL spikevax™ bivalent Original/Omicron BA.4/BA.5 dispersion for injection bivalent COVID-19 mRNA Vaccine IM Multidose vial 2.5 mL
LOT EXP |
*Note: The 2-D matrix code may not appear on all vial labels with the SPIKEVAX™ Bivalent Original/Omicron BA.4/BA.5 brand name.
Carton Label

|
Moderna spikevax™ bivalent Original / Omicron BA.4/BA.5 dispersion for injection bivalent COVID-19 mRNA Vaccine (nucleoside modified) Intramuscular use.
|
0.1 mg/mL 10 multidose vials each vial contains 2.5 mL |
|
Black box contains: PC EXP LOT
Moderna™ Moderna Biotech Spain, S.L. Calle del Principe de Vergara 132 Plt 12, Madrid 28002, Spain |
Each multidose vial contains 2.5 mL.
Store frozen at -50°C to -15°C. Read the package leaflet for the shelf life after first opening and for additional storage information. Keep out of sight and reach of children. Dispose of in accordance with local requirements.
Read the package leaflet before use. Scan here for package leaflet or visit www.ModernaCOVID-19Global.com [QR code] |
*Note: The 2-D matrix code may not appear on all carton labels with the SPIKEVAX™ Bivalent Original/Omicron BA.4/BA.5 brand name.
Additional information
Details
Get notified
Receive notifications for new and updated recalls and alerts by category.