This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
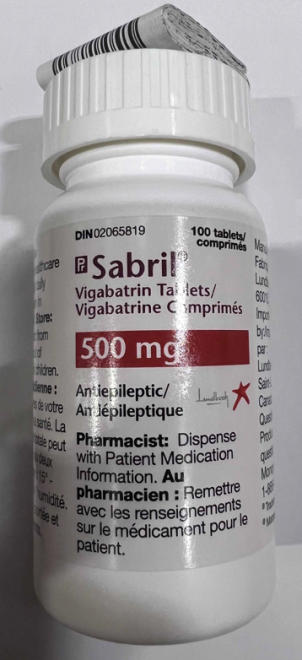
Sabril (vigabatrin) 500 mg sachets and tablets found to contain trace amounts of another drug
Summary
Do not stop taking Sabril without consulting your health care professional as stopping this drug comes with serious risks. Unless you have a severe allergy to tiapride, the benefits of this drug are expected to continue to outweigh its potential risks despite the presence of trace amounts of tiapride. Monitor for any new and unexpected side effects. Seek immediate medical attention of you experience symptoms of a severe allergic reaction, such as difficulty breathing or swallowing or severe swelling of the lips, mouth or throat. Consult your health care professional if you have any concerns.
Affected products
| Product | DIN | Lot number | Expiry date | Date added |
|---|---|---|---|---|
| Sabril (vigabatrin) 500 mg tablets | 02065819 | 3216947 | Mar-2026 | 2023-11-09 |
| Sabril (vigabatrin) 500 mg sachets | 02068036 | Lot 3209710A | Aug-2026 | 2023-09-19 |
| Sabril (vigabatrin) 500 mg sachets | 02068036 | Lot 3214708A | Feb-2027 | 2023-08-18 |
| Sabril (vigabatrin) 500 mg sachets | No DIN on label (this is a U.S.-labelled lot imported to help address a current shortage) | Lot 3207333A | Mar-2027 | 2023-08-18 |
Issue
UPDATE – November 9, 2023:
Further to the advisory below, in discussion with Health Canada, Lundbeck Canada Inc. is releasing one lot of Sabril (vigabatrin) 500 mg tablets on the Canadian market found to contain trace amounts of another prescription drug, tiapride. This lot is in addition to the lots of Sabril in sachet format described below. The tablets are being released given the current shortage and no immediate available alternative supply of tablets. Unless you have a severe allergy to tiapride, the benefits of this drug are expected to continue to outweigh its potential risks despite the presence of trace amounts of tiapride. Patients are advised to not stop taking Sabril without consulting their health care professional, as stopping this drug comes with serious risks.
To help mitigate the ongoing shortage, Health Canada is working with stakeholders to monitor the supply of sachets and tablets. Recently, Health Canada permitted the importation of foreign-authorized vigabatrin sachets to minimize impacts of the shortage. Health Canada has also permitted the importation of foreign-authorized tablets and is working with the importer to determine when they will be available at pharmacies.
The above-mentioned release of one lot of Sabril (vigabatrin) 500 mg tablets on the Canadian market will help meet demand for the product until additional supply is available. See the original advisory below for more information.
UPDATE – September 19, 2023:
Further to the advisory below, in discussion with Health Canada, Lundbeck Canada Inc. is releasing an additional lot Sabril (vigabatrin) 500 mg in sachet format on the Canadian market found to contain trace amounts of another prescription drug, tiapride.
Original Advisory – August 18, 2023:
Lundbeck Canada Inc. has informed Health Canada that two lots of Sabril (vigabatrin) 500 mg powder for oral solution, in sachet format, were found to contain trace amounts of another prescription drug, tiapride.
Sabril is a prescription drug used to manage epileptic seizures in adults and children, and for infantile spasms (West Syndrome).
Health Canada has determined that the health risk of the trace contamination to patients is low. It is important that patients do not stop taking their Sabril as prescribed without consulting their physician, as the benefits of this drug are expected to outweigh its potential risks despite trace amounts of tiapride, unless patients have a severe allergy to tiapride. Sabril therapy should not be interrupted, as this may increase the risk of seizures, which could be life-threatening.
There are no authorized products containing tiapride in Canada. In some other countries, tiapride is a drug used to treat a range of neurological and psychiatric disorders including dyskinesia (involuntary movements), alcohol withdrawal syndrome, psychosis, agitation and aggression in the elderly, and tic disorders in children.
Lundbeck Canada Inc., the Canadian manufacturer of Sabril, is reporting ongoing shortages of this medication and no alternative supply is available in Canada at this time. As a result, Health Canada is not recommending that the company recall this medication at this time. Health Canada continues to monitor the situation.
Health Canada recognizes how important Sabril is for the people taking it. Health Canada is leading work with provincial and territorial governments, manufacturers and stakeholders across the supply chain and healthcare system to mitigate the ongoing shortage of Sabril. This includes accessing additional foreign-authorized supply, where possible.
Although this drug is not being recalled, Health Canada is monitoring the company's implementation of any necessary corrective and preventative actions to prevent this from happening again.
What you should do
- Do not stop taking your Sabril medication without consulting your physician.
- Monitor for any new and unexpected side effects. Seek immediate medical attention of you experience symptoms of a severe allergic reaction, such as difficulty breathing or swallowing or severe swelling of the lips, mouth or throat.
- Consult your health care professional if you or your child are taking Sabril and you have any concerns.
- Report any health product-related side effects or complaints to Health Canada.
Additional information
Details
Media and public enquiries
Media Enquiries:
Health Canada
(613) 957-2983
media@hc-sc.gc.ca
Public Enquiries:
(613) 957-2991
1-866 225-0709
info@hc-sc.gc.ca
Get notified
Receive emails about new and updated recall and safety alerts.