One lot of Pharmasave Advanced Relief Eye Drops and Compliments Advanced Relief Eye Drops recalled because of a packaging error that may pose health risks
Summary
Do not use the affected product. Consult a health care professional if you have used this product and have health concerns.
Affected products
| Product | DIN | Lot | Expiry |
|---|---|---|---|
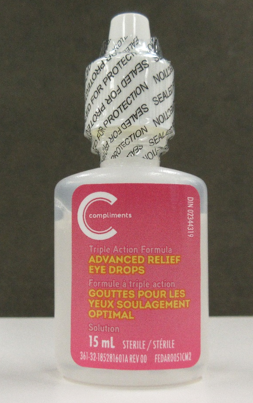
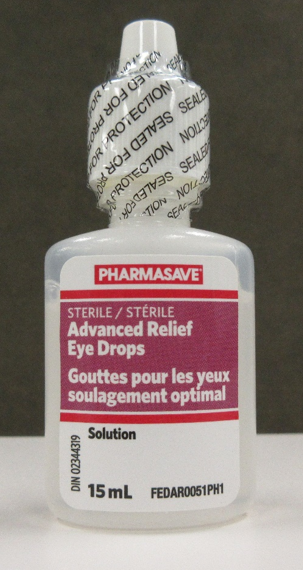
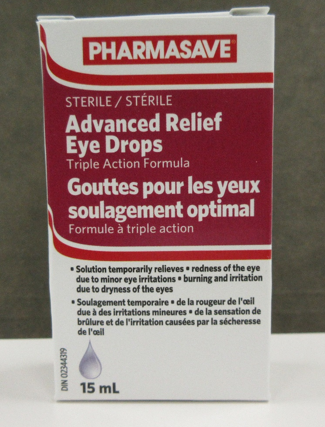
| Pharmasave Advanced Relief Eye Drops, 15 mL | 02344319 | AR21C03 (on cartons and some bottles) RL21D01 (on some bottles) | 31 Mar 2024 |
| Compliments Advanced Relief Eye Drops, 15 mL | 02344319 | AR21C03 (on cartons and some bottles) RL21D01 (on some bottles) | 31 Mar 2024 |
Issue
Teva Canada Ltd. is recalling one lot each of Pharmasave Advanced Relief Eye Drops and Compliments Advanced Relief Eye Drops because of a packaging error. Some bottles may contain ingredients that are not listed on the label and that may pose health risks to individuals who are allergic to the ingredients.
The products are used for the temporary relief of eye redness due to minor eye irritation, burning and dryness. They are labelled for use by adults and children 6 years of age and older.
Following a complaint involving the Pharmasave eye drops, the company identified that some bottles in one lot of both the Pharmasave and Compliments products may have been packaged with another eye-drop product that contains different active ingredients. Glycerine 0.25% and naphazoline HCl 0.012% were present in bottles which were labelled to contain Dextran 70 0.1%, Povidone 1%, Polyethylene glycol 400 1%, Tetrahydrozoline hydrochloride 0.05%.
Naphazoline HCl or glycerine may cause a reaction in individuals allergic to these ingredients. Signs of an allergic reaction include rash, and itching or swelling, especially of the face, tongue and throat.
Cartons of the affected Pharmasave and Compliments eye drops are labelled with lot number AR21C03. The affected bottles may be labelled with either lot number AR21C03 or RL21D01.
Health Canada is monitoring the company's recall and implementation of any necessary corrective and preventative actions. If additional safety information is identified, Health Canada will take appropriate action and inform Canadians as needed.
What you should do
- Do not use the affected product. Consult a health care professional if you have used this product and have health concerns.
- Contact Teva Canada Ltd. at Teva Canada Customer Care by calling 1-800-268-4129 if you have questions about the recall.
- Report any health product-related side effects or complaints to Health Canada.
Additional information
Details
Media and public enquiries
Media Enquiries:
Health Canada
(613) 957-2983
media@hc-sc.gc.ca
Public Enquiries:
(613) 957-2991
1-866 225-0709
info@hc-sc.gc.ca
Get notified
Receive emails about new and updated recall and safety alerts.