Matrix-Brand Training Cycles by Johnson Health Tech North America recalled due to potential fall hazard
Summary
Immediately stop using the recalled products and contact Johnson Health Tech North America Inc. ("JHTNA") to schedule a service technician to install a free repair kit.
Affected products
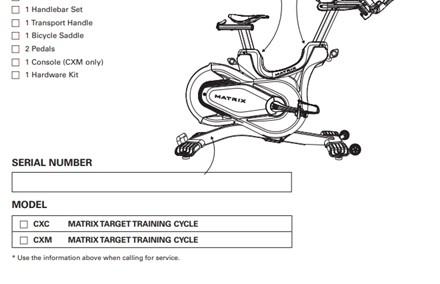
This recall involves certain Matrix-brand Training Cycles models CXP-03, CXC-02, CXM-03, CXV and CXM-02. The affected products have adjustable saddles and handlebars with an aluminum rear flywheel and a magnetic resistance system. They have matte black steel frames, forged steel cranks, dual-sided SPD pedals, four leveling feet and two transport wheels. "Matrix" is printed on the side. The model and serial numbers are located on a label on the lower rear area of the cycle frame. Recalled cycles have a serial number that contains a serial prefix of FC32, ZFC32B, FC33, FC36, FC29D or FC27.
| Model (Serial Prefix) | Example Serial Numbers |
|---|---|
| CXC-02 (FC32) | FC32210900001 |
| CXC-02 (ZFC32) | ZFC32B240600001 |
| CXM-02 (FC33) | FC33210800001 |
| CXM-03 (FC36) | FC36240300001 |
| CXP-03 (FC29) | FC29D240600001 |
| CXV (FC27) | FC27210700001 |
Issue
The training cycles’ adjustable seat can unexpectedly lower while in use, posing a potential fall hazard to consumers.
As of January 22, 2025, the company has received no reports of incident or injury in Canada. In the United States, the company has received 63 reports of seats unexpectedly lowering, including two reports that users fell off the cycle when the seat lowered. No injuries have been reported in the United States.
What you should do
Consumers should immediately stop using the recalled cycles and contact JHTNA to schedule a service technician to install a free repair kit.
For more information, consumers can contact JHTNA toll-free at 866-218-3674, from 8 a.m. to 5 p.m. CT, Monday through Friday, by email at cxrecall@matrixfitness.com or online at https://www.matrixfitness.com/us/eng/recalls, or go to www.matrixfitness.com and click on "Recalls". The company is contacting all known purchasers directly.
Joint recall with Health Canada, the United States Consumer Product Safety Commission (US CPSC) and Johnson Health Tech North America.
Please note that the Canada Consumer Product Safety Act prohibits recalled products from being redistributed, sold or even given away in Canada.
Health Canada would like to remind consumers to report any health or safety incidents related to the use of this product or any other consumer product or cosmetic by filling out the Consumer Product Incident Report Form.
This recall is also posted on the OECD Global Portal on Product Recalls website. You can visit this site for more information on other international consumer product recalls.
Additional information
Background
Number Sold
The company reported that 140 units of the affected product were sold in Canada (exclusively to commercial purchasers, such as fitness facilities) and approximately 12,885 units were sold in the United States.
Time Period Sold
The affected products were sold from January 2021 to October 2024.
Place of Origin
Manufactured in Taiwan
Details
Manufacturer
Johnson Health Tech North America Inc.
Cottage Grove, Wisconsin
United States
Get notified
Receive emails about new and updated recall and safety alerts.