Labelling error may lead to patients receiving Linessa 28 birth control pills instead of Linessa 21
- Starting date:
- June 12, 2019
- Type of communication:
- Advisory
- Subcategory:
- Drugs
- Source of recall:
- Health Canada
- Issue:
- Labelling and Packaging, Important Safety Information
- Audience:
- General Public
- Identification number:
- RA-70187
Last updated: 2019-06-12
- Issue
- Products affected
- What you should do
- Who is affected
- Background
- Media enquiries
- Public enquiries
- What Health Canada is doing
- Images
Issue
OTTAWA - Health Canada is advising Canadians of a labelling error affecting one lot of Linessa 28 birth control pills on the Canadian market (lot 190056, expiry 06/2021). The English side of Linessa 28 boxes is incorrectly labelled with the eight-digit Drug Identification Number (DIN) for Linessa 21 (02272903) instead of the correct Linessa 28 DIN (02257238). The labelling error could result in a Linessa 21 patient inadvertently receiving Linessa 28 instead of Linessa 21 if the product is dispensed at the pharmacy only using the incorrect DIN as a reference.
There is a small chance that patients who were prescribed Linessa 21 but received Linessa 28 in error may not know that the last 7 green "reminder" pills in the Linessa 28 pack are inactive pills that do not contain any hormones. If patients take a "break" week (no pills) after completing a 28-day pack, as is the practice with Linessa 21, this would result in two weeks without hormone treatment, which could increase the risk of pregnancy.
The DIN error affects the English side of Linessa 28 boxes only. The French side of Linessa 28 boxes has the correct DIN. The product name "Linessa 28" is clearly and accurately printed on the box, the foil pouch and the blister pack.
The labelling issue does not affect the safety or quality of Linessa pills. Patients who take Linessa 21 or Linessa 28 according to the instructions that accompany the product they received are not at an increased risk of pregnancy as a result of the labelling error.
At Health Canada's request, Aspen Pharmacare Canada Inc. has asked that pharmacies contact patients prescribed Linessa 21 to confirm that they received the correct product.
According to the company, approximately 10,000 mislabelled packages from lot 190056 have been distributed nationally, starting in late May 2019. A second lot of Linessa 28 is affected by the same issue (lot 190141, expiry 06/2021) but has not yet been released on to the market. A sticker with the correct DIN will be placed on all affected Linessa 28 boxes that have not yet been distributed, to prevent patients from receiving Linessa 28 instead of Linessa 21.
Products affected
- Linessa 28, lot 190056, expiry 06/2021
- Linessa 28, lot 190141, expiry 06/2021
What you should do
-
If you were prescribed Linessa 21 but received Linessa 28, or if you are unsure, contact your pharmacy to confirm that you have the product that was prescribed for you and that you understand the directions for use.
- You do not need to return your packages of Linessa 28 to the pharmacy unless advised to do so by your pharmacist. There is no increased risk of pregnancy if you are taking Linessa 21 or Linessa 28 according to the instructions that accompany the product you received. Instructions are also available from the company's website (click the Patient Information button to download the instructions).
- Contact your pharmacy if you have any questions.
- If you were prescribed Linessa 28 and received Linessa 28, continue to take the pills as directed in the product instructions. Contact your pharmacy if you have any questions. You may receive packages of Linessa 28 with a sticker indicating the correct DIN.
- If you were prescribed Linessa 21 and received Linessa 21, continue to take the pills as directed in the product instructions. Contact your pharmacy if you have any questions.
- Report any health product-related adverse reactions or complaints to Health Canada.
Who is affected
- Patients prescribed Linessa 21 but who received Linessa 28
Background
Linessa 21 and Linessa 28 are prescription drugs used to prevent pregnancy.
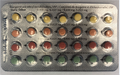
Linessa 21 contains 21 active pills (with hormones) [7 light yellow, 7 orange and 7 red] taken daily for three weeks (followed by a week of no pills unless otherwise directed by the prescriber).
Linessa 28 contains 21 active pills (with hormones) [7 light yellow, 7 orange and 7 red] taken daily for three weeks, and then seven (7) [green] "reminder" pills (without hormones) taken daily for one week.
Media enquiries
Health Canada
613-957-2983
hc.media.sc@canada.ca
Public enquiries
613-957-2991
1-866-225-0709
What Health Canada is doing
Health Canada continues to follow up with the company to make sure that appropriate risk mitigation measures are taken. We will continue to monitor and assess the need for further action, and will update Canadians as necessary.
Images
Select thumbnail to enlarge - opens in a new window