Importation of US-Authorized Fludarabine Phosphate Injection, USP due to the Current Shortage of Canadian-Authorized Fludarabine Phosphate Injection
Summary
See Key Messages below
Affected products
| Brand name | Dosage form and route of administration | Country of authorization and identifying code | Manufacturer | Importer in Canada |
|---|---|---|---|---|
| Fludarabine Phosphate Injection, USP 50mg/2mL (25 mg/mL) | Solution Intravenous | US NDC: 59923-604-02 | Areva Pharmaceuticals, Inc. | Septa Pharmaceuticals Inc. |
Issue
There is a shortage of Fludarabine Phosphate Injection in Canada. Given the medical necessity of Fludarabine Phosphate Injection and the need to maintain continuity of supply in Canada, Health Canada has authorized the exceptional, temporary importation and sale of US-authorized Fludarabine Phosphate Injection, USP with English-only labels, by Septa Pharmaceuticals Inc.
Healthcare professionals should be aware of important differences between the US-authorized and Canadian-authorized products (see Information for healthcare professionals section).
Audience
Healthcare professionals including hematologists, medical oncologists, transplant medicine/ hematopoietic stem cell transplant groups, family physicians, and hospital pharmacists.
Key messages
- Due to a shortage of Fludarabine Phosphate Injection in Canada, and given the medical necessity of this drug, Health Canada has permitted the exceptional, temporary importation and sale of US-authorized Fludarabine Phosphate Injection, USP with English-only labels.
- This US-authorized product has the same active ingredient (fludarabine phosphate), strength (25 mg/mL), dosage form (injection), and route of administration (intravenous) as the Canadian-authorized product. However, there are important differences between the US-authorized and the Canadian-authorized products.
- Healthcare professionals are advised to:
- Be aware of important differences in storage and stability (see the Information for healthcare professionals section) between the US-authorized and the Canadian-authorized Fludarabine Phosphate Injection products.
- Be aware that there are important instructions that should be followed for preparation and stability of the prepared solution for infusion, which are supported by compatibility studies (see the Information for healthcare professionals section). These instructions are NOT found in the US product labelling or package insert and they differ from the Canadian product labelling.
- Refer to the Canadian Product Monograph for Fludarabine Phosphate Injection, USP by Accord Healthcare Inc. (DIN 02438577), available in English and French on Health Canada’s Drug Product Database, for information on authorized indications, contraindications, dosage, special handling instructions and other safety information.
Background
In Canada, Fludarabine Phosphate Injection is indicated for the second line treatment of adult patients with chronic lymphocytic leukemia (CLL) and low-grade non-Hodgkin’s lymphoma (Lg-NHL) who have failed other conventional therapies.
Currently, there is a shortage of Canadian-authorized Fludarabine Phosphate Injection in Canada. To help mitigate the shortage, Health Canada has permitted the exceptional, temporary importation and sale of US-authorized Fludarabine Phosphate Injection, USP, with English-only labels.
Information for consumers
Fludarabine Phosphate Injection is indicated for the second line treatment of adult patients with chronic lymphocytic leukemia (CLL) and low-grade non-Hodgkin's lymphoma (Lg-NHL) when other treatments have not worked.
Patients should discuss any questions or concerns about this information with their healthcare professional. Patients should continue to inform their healthcare professional if they are experiencing any side effects while receiving fludarabine phosphate.
Information for healthcare professionals
The US-authorized Fludarabine Phosphate Injection, USP has the same active ingredient, strength (25 mg/mL), dosage form (injection), and route of administration (intravenous) as the Canadian-authorized product. However, the US-authorized product has some important differences from the Canadian-authorized product.
Healthcare professionals are advised of the following:
- There are additional storage instructions included on the vial label of the US-authorized product (see images of the US-authorized product in Appendix 1) including: protect from light and retain the unopened vial in the carton.
- The US product, like the Canadian product, contains no antimicrobial preservative. Although not stated on the US label, the unused portion should be discarded after vial puncture, as per the Canadian Product Monograph.
- Be aware that there are important instructions that should be followed for preparation and stability of the prepared solution for infusion, which are supported by compatibility studies. These instructions are NOT found in the US product labelling or package insert and they differ from the Canadian product labelling:
- Be aware that the product must be further diluted for intravenous infusion administration in PVC bags with a concentration between 0.4 mg/mL and 1 mg/mL in 5% Dextrose Injection USP, or in 0.9% Sodium Chloride Injection USP.
- Following dilution, the prepared solution should be used within 8 hours when kept at room temperature or when refrigerated (2°C to 8°C).
- Refer to the Canadian Product Monograph for Fludarabine Phosphate Injection, USP by Accord Healthcare Inc. (DIN 02438577), available in English and French on Health Canada’s Drug Product Database, for information on authorized indications, contraindications, dosage, special handling instructions and other safety information.
Take care to ensure chemical integrity and sterility of infusion solutions are maintained. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Fludarabine Phosphate Injection should not be mixed with other drugs.
Proper selection of the intended product must be verified to avoid confusion with other products and to prevent medication errors.
The US-authorized product does not have a Drug Identification Number (DIN) or a barcode that scans in medication management systems in Canada. A facility-generated sticker may be required to enable barcode scanning and allow the product being dispensed and administered to be properly identified.
Action taken by Health Canada
To help mitigate the shortage of Fludarabine Phosphate Injection in Canada, Health Canada has permitted the exceptional, temporary importation and sale of US-authorized Fludarabine Phosphate Injection, USP and has added this product to the List of Drugs for Exceptional Importation and Sale.
Health Canada has worked with Septa Pharmaceuticals Inc. to prepare this alert for Fludarabine Phosphate Injection, USP. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication will be further distributed through the MedEffect™ e-Notice email notification system.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any case of serious or unexpected side effects in patients receiving US-authorized Fludarabine Phosphate Injection, USP 25 mg/mL should be reported to Septa Pharmaceuticals Inc. or Health Canada.
Septa Pharmaceuticals Inc.
7035 Maxwell Road, Unit 2
Mississauga, ON
L5S 1R5
Canada
Telephone: +1 905-564-5665
Fax: +1 905-564-1291
To correct your mailing address or fax number, contact Septa Pharmaceuticals Inc.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting (http://www.hc-sc.gc.ca/dhp-mps/medeff/report-declaration/index-eng.php) for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Regulatory Operations and Enforcement Branch
E-mail: hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Original signed by
Devinder Kumar
President and CEO
Appendix 1
US-authorized Fludarabine Phosphate Injection, USP 25 mg/mL, outer carton:
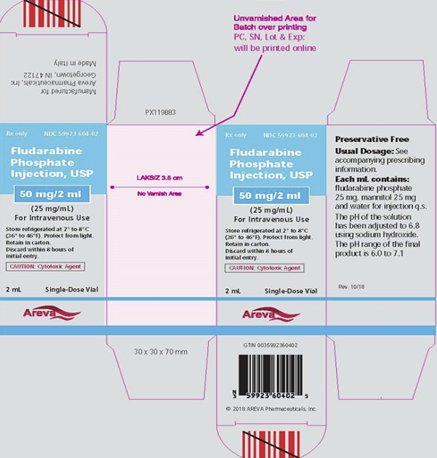
NDC 59923-604-02
Fludarabine Phosphate Injection, USP
50 mg/2 mL
(25 mg/mL)
For Intravenous Use
Store refrigerated at 2° to 8°C
(36° to 46°F). Protect from light.
Retain in carton.
Discard within 8 hours of initial entry.
CAUTION: Cytotoxic Agent
2 mL
Single-Dose Vial
Preservative Free
Usual Dosage: See Accompanying Prescribing Information
Each mL contains:
Fludarabine phosphate
25mg, mannitol 25mg
and water for injection q.s.
The pH of the solution has been adjusted to 6.8 using sodium hydroxide.
The PH range of the final product is 6.0 to 7.1
Rev 10/18
US-authorized Fludarabine Phosphate Injection, USP 25 mg/mL, vial label:

Rx only
NDC 59923-604-02
Fludarabine Phosphate Injection, USP
50 mg/2 mL
(25 mg/mL)
Caution: Cytotoxic Agent
For Intravenous Use
2 mL
Single-Dose Vial
P2119832
Usual Dosage: See accompanying prescribing information.
Store refrigerated at 2° to 8°C
(36° to 46°F). Protect from light.
Retain in carton. Discard within 8 hours of initial entry.
Manufactured for:
Areva Pharmaceuticals, Inc
Georgetown, IN 47122
Made in Italy
Lot:
Exp:
US-authorized Fludarabine Phosphate Injection, USP 25 mg/mL, vial:

Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.