This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Important Safety Information on Xylocaine (Lidocaine HCI) Jelly 2% Single Use Plastic Syringe (10 mL): Update Notice to Hospitals
- Starting date:
- July 20, 2007
- Posting date:
- August 7, 2007
- Type of communication:
- Notice to Hospitals
- Subcategory:
- Drugs
- Source of recall:
- Health Canada
- Audience:
- Healthcare Professionals
- Identification number:
- RA-170001740
This is duplicated text of a letter from AstraZeneca Canada Inc.
Contact the company for a copy of any references, attachments or enclosures.
Notice about Health Canada advisories
NOTICE TO HOSPITALS - UPDATE - Health Canada Endorsed Important Safety Information on Xylocaine (lidocaine HCl) Jelly 2% Single Use Plastic Syringe (10 mL) Product No. 063, DIN 00385484
July 20, 2007
Dear Pharmacy Director,
Please distribute to the Chief of Staff, Department of Nursing, Department of Urology, Department of Surgery, Department of Emergency Medicine, Department of Intensive Care, Department of Respirology, Department of Internal Medicine, Department of Ophthalmology and other professional staff that might administer this product and POST THIS NOTICE and current Handling Instructions (Version # 61063, Date: 11/2006) in appropriate locations in your hospital.
Subject: Xylocaine® (lidocaine HCl) Jelly 2% Single Use Plastic Syringe (10 mL) without preservative
On September 22, 2006, AstraZeneca recalled Xylocaine® (lidocaine HCl) Jelly 2% Single Use Plastic Syringe (10 mL) because of an issue related to the break seal construction of this syringe product and how it was being opened. On September 27, 2006 Health Canada issued a Public Advisory that also described the potential risk. Due to the lack of appropriate alternative options in some situations, as identified to Health Canada by hospitals, it became apparent that there is a continuing need for this product for in-hospital use only.
As indicated in the November 29, 2006 Notice to Hospitals, following consultations between AstraZeneca and Health Canada, it has been concluded that the potential risk can be managed by users following the attached current Handling Instructions.
Effective December 5, 2006, the above product was made available for use in hospitals for indicated procedures where there were no appropriate alternatives. This product was restricted to in-hospital use only and was not given to patients for use in the home. These restrictions were to be in place until a proposed new Xylocaine® Jelly 2% syringe became available, which was anticipated to happen in approximately May 2007.
Since November 2006, AstraZeneca has voluntarily explored alternative designs for the break seal construction of the syringe but did not find a feasible alternative design that offered improvement. As such, the existing syringe design will continue to be available, subject to the above restrictions and the current Handling Instructions. AstraZeneca has revised the Prescribing Information to include the current Handling Instructions and will be incorporating the current Handling Instructions onto the Xylocaine® Jelly 2% syringe carton and blister.
Please ensure that:
- all hospital healthcare professionals administering this product are informed of the potential risks identified in the Health Canada Advisory dated September 27, 2006.
- all hospital healthcare professionals administering this product follow the current Handling Instructions attached to this Product Update and enclosed with the product to avoid the potential risk.
Please note that all orders for the product must be placed directly with AstraZeneca Customer Relations at 1-800-668-6000.
Xylocaine® (lidocaine HCl) Jelly 2% Single Use Plastic Syringe (10 mL) should be utilized under sterile techniques and is only indicated for surface anesthesia and lubrication for:
- The male and female urethra during cystoscopy, catheterization exploration by sound and other endourethral operations
- Nasal and pharyngeal cavities in endoscopic procedures such as gastroscopy and bronchoscopy
- Proctoscopy and rectoscopy
- Tracheal intubation
This product is also indicated for symptomatic treatment of pain in connection with cystitis and urethritis.
Managing marketed health product-related adverse reactions depends on healthcare professionals and consumers reporting them. Reporting rates determined on the basis of spontaneously reported post-market adverse reactions are generally presumed to underestimate the risks associated with health product treatments. Any suspected adverse reactions in patients receiving Xylocaine® Jelly 2% should be reported to AstraZeneca or Health Canada at the following addresses:
AstraZeneca Canada Inc.
1004 Middlegate Road
Mississauga, Ontario L4Y 1M4
Tel: 1-800-668-6000
Fax: 1-800-267-5743
Any suspected adverse reaction can also be reported to:
Canadian Adverse Drug Reaction Monitoring Program (CADRMP)
Marketed Health Products Directorate
HEALTH CANADA
Address Locator: 0701C
OTTAWA, Ontario, K1A 0K9
Tel: 613-957-0337 or Fax: 613-957-0335
To report an Adverse Reaction, consumers and health professionals may call toll free:
Tel: 866-234-2345
Fax: 866-678-6789
cadrmp@hc-sc.gc.ca
The AR Reporting Form and the AR Guidelines can be found on the Health Canada web site or in The Canadian Compendium of Pharmaceuticals and Specialties.
For other inquiries related to this communication, please contact Health Canada at:
Bureau of Cardiology, Allergy and Neurological Sciences (BCANS)
E-mail: bcans_enquiries@hc-sc.gc.ca
Tel.: 613-941-1499
Fax: 613-941-1668
original signed by
Karen Feltmate
Vice President, Regulatory Affairs
AstraZeneca Canada Inc.
Xylocaine® and the AstraZeneca logo are trade-marks of the AstraZeneca group of companies.
Please post
Handling Instructions for Xylocaine® Jelly 2% (lidocaine hydrochloride) Single Use Plastic Syringe (10mL)
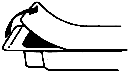
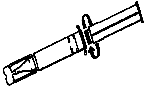
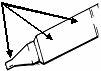
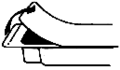
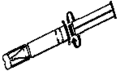
1. Tear off the paper cover.
2. Screw the plunger rod clockwise into grey rubber until rubber rotates.
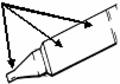
3. Twist the protective tab and then, without bending, pull slightly to break the seal.
4. Extrude a small amount (i.e. 1 cm) of Jelly. Inspect the syringe to ensure that there is no plastic fragment present in the Jelly.
Note: Upon Visual Inspection, A Clear Plastic Fragment In Clear Jelly May Be Difficult To Detect And May Look Like An Air Pocket.
If the protective tab is broken, do not use the syringe.
5. The syringe must not be used and must be discarded if there is any suspicion of a broken plastic fragment.
6. If the protective tab is intact and no plastic fragment is found in the Jelly, the syringe is ready for use.
For further assistance please contact AstraZeneca Canada at 1-800-668-6000.
(Version # 61063, Date: 11/2006)
Images
Select thumbnail to enlarge - opens in a new window