Horizon T101-05 Folding Treadmills recalled due to fall hazard
Brand(s)
Summary
Immediately stop using the product and contact Horizon Fitness for more information and to obtain updated software.
Affected products
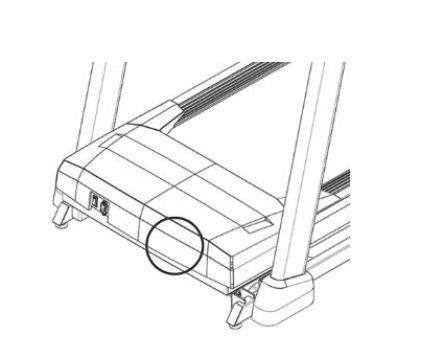
This recall involves Horizon Fitness T101-05 folding treadmills. The Treadmills have a running area that measures 55 inches long by 20 inches wide and have a 33-inch-wide console. The model name and the serial number are located on the front of the motor cover near the power switch. The serial number starts with the letters “TM”. The recalled Treadmills have serial numbers starting with either TM734 or TM486.
| MODEL NAME | AFFECTED FRAME SERIAL NUMBERS |
|---|---|
| Frame Production Code – TM734 | TM734XXXX00501 |
| Frame Production Code – TM486 | TM486XXXX00501 |
Issue
The Treadmills can unexpectedly change speed, posing a risk that a consumer will fall.
As of August 25, 2022, the company has received 8 reports of units unexpectedly changing speed in Canada, including 6 reports of injuries. In the United States, the company has received 874 reports of units unexpectedly changing speed, including 71 reports of injuries.
What you should do
Consumers should stop using the treadmills and contact Horizon Fitness to obtain updated software.
Consumers can contact Horizon Fitness online by email at retailrecall@johnsonfit.com or www.horizonfitness.com and click on “Safety Notices.” Consumers can also contact Horizon by telephone at 1-888-223-1045 from 8 a.m. to 5 p.m. CT Monday through Friday.
Joint recall with Health Canada, the United States Consumer Product Safety Commission (US CPSC) and Johnson Health Tech Trading, Inc.
Please note that the Canada Consumer Product Safety Act prohibits recalled products from being redistributed, sold or even given away in Canada.
Health Canada would like to remind Canadians to report any health or safety incidents related to the use of this product or any other consumer product or cosmetic by filling out the Consumer Product Incident Report Form.
This recall is also posted on the OECD Global Portal on Product Recalls website. You can visit this site for more information on other international consumer product recalls.
Additional information
Background
Number Sold
The company reported that 6,986 units of the affected product were sold in Canada and approximately 192,000 were sold in the United States.
Time Period Sold
The affected products have been sold in Canada from August 2018 to October 2022.
Place of Origin
Manufactured in China and Vietnam
Details
Distributor
Johnson Health Tech Trading, Inc.
Cottage Grove, Wisconsin
U.S.A.
Manufacturers
Johnson Industries Co., Ltd.
Shanghai, China
Johnson Health Industry (Vietnam) Company Ltd.
Vietnam
Get notified
Receive emails about new and updated recall and safety alerts.