Health Canada seized unauthorized Bielenda Dr. Medica anti-acne products because they may pose serious health risks
- Starting date:
- September 3, 2019
- Type of communication:
- Advisory
- Subcategory:
- Drugs
- Source of recall:
- Health Canada
- Identification number:
- RA-70893
Last updated:
- What you should do
- Who is affected
- Background
- Media enquiries
- Public enquiries
- What Health Canada is doing
- Images
OTTAWA – Health Canada is advising consumers that Bielenda Dr. Medica anti-acne day/night cream and toning liquid are unauthorized products and may pose serious health risks. According to the product labels, they contain a prescription drug (azelaic acid or an azelaic acid derivative).
Health Canada seized the unauthorized anti-acne cream from the retail store, The Deli Corner (Oshawa, ON), and its distributor, MAP International (Oakville, ON). Health Canada also seized the unauthorized anti-acne toning liquid from the distributor.
Selling unauthorized health products in Canada is illegal. Health products that have not been authorized by Health Canada have not been assessed for safety, effectiveness and quality, and may pose serious health risks. For example, unauthorized health products may be contaminated, contain dangerous ingredients not listed on the label, or not contain the ingredient shown on the label.
Prescription drugs should be taken only under the advice and supervision of a healthcare professional because they are used to treat specific conditions and may cause serious side effects.
What you should do
- Stop using these products. Consult your health care professional if you have used any of these products and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products are currently authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Products Database.
- Report any health product adverse events or complaints to Health Canada.
- Stay connected with Health Canada and receive the latest advisories and product recalls.
Who is affected
Consumers who have bought or used any of the affected products.
| Photo | Product | Risk | Seized from |
|---|---|---|---|
| Bielenda Dr. Medica dermatological anti-acne day/night cream | Labelled to contain an azelaic acid derivative |
The Deli Corner
MAP International |
|
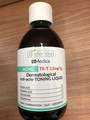
| Bielenda Dr. Medica dermatological anti-acne toning liquid | Labelled to contain azelaic acid |
MAP International 2733 Coventry Rd, Oakville, ON |
Background
Azelaic acid is a prescription product used to treat rosacea (unusual redness of the skin). It should not be used by people who are allergic to azelaic acid, propylene glycol or benzoic acid. It may cause skin irritation (itch, burning, stinging, reddening, or scaling), hypersensitivity (swelling of the face or eyes, shortness of breath, or hives) or changes in skin color. Women who are or are planning to become pregnant or to breastfeed should consult with their doctor or pharmacist prior to using azelaic acid. Contact with the eyes and mouth should be avoided, and young children should not touch the treated area because of its irritant effect.
Media enquiries
Health Canada
(613) 957-2983
hc.media.sc@canada.ca
Public enquiries
(613) 957-2991
1-866 225-0709
What Health Canada is doing
Health Canada seized the products from the retail store and the distributor. Health Canada is working with the Canada Border Services Agency to help prevent the importation of these products. Should additional safety concerns be identified, Health Canada will take appropriate action and inform Canadians as necessary.
Images
Select thumbnail to enlarge - opens in a new window