This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Foreign Product Alerts: Steelman Capsules 2, CO Feng Shi Gu Tong Ning Jiao Nang, and Fu Fang Feng Shi Gu Kang Ling Jiao Nang
- Starting date:
- August 7, 2013
- Posting date:
- August 7, 2013
- Type of communication:
- Foreign Product Alert (FPA)
- Subcategory:
- Natural health products
- Source of recall:
- Health Canada
- Issue:
- Undeclared Substance
- Audience:
- General Public
- Identification number:
- RA-34861
Introduction
These products are not authorized for sale in Canada and have not been found in the Canadian marketplace, but it is possible they may have been brought into the country by travellers or purchased over the Internet.
Affected products
- Steelman Capsules 2
- CO Feng Shi Gu Tong Ning Jiao Nang
- Fu Fang Feng Shi Gu Kang Ling Jiao Nang
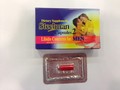
Steelman Capsules 2
Product description
This product is promoted for "libido concerns"
Hazard identified
The U.S.The Hong Kong Department of Health advised consumers not to use this product after it was found to contain undeclared aminotadalafil.
- Aminotadalafil is an unauthorized substance similar to tadalafil and may pose similar health risks. Tadalafil is a prescription drug used to treat erectile dysfunction and should only be taken under the supervision of a health care practitioner.
Place of origin
This product is manufactured by Ocvita Labs LLC, based in the United States. It is distributed in Hong Kong by Top Harvest Pharmaceutical Company.
Side effects
Tadalafil should not be used by individuals taking any kind of nitrate drug (e.g. nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heart beat. Other side-effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
CO Feng Shi Gu Tong Ning Jiao Nang
Product description
This product is promoted for joint pain.
Hazard identified
The Hong Kong Department of Health advised consumers not to use this product after it was found to contain undeclared prednisone, diclofenac, ibuprofen, hydrochlorothiazide, metoclopramide and trimethoprim.
- Prednisone is a steroidal prescription drug used to treat inflammatory conditions such as arthritis and allergic reactions.
- Diclofenac is a prescription drug in the family known as non-steroidal anti-inflammatory drugs (NSAIDs) that are used to treat pain, fever and inflammation.
- Ibuprofen is a non-prescription NSAID.
- Hydrochlorothiazide is a diuretic (water pill) prescription drug used to remove excess fluid from the body.
- Metoclopramide is a prescription drug used to aid stomach emptying.
- Trimethoprim is a prescription antibiotic mainly used in the treatment of urinary tract infections.
Prescription drugs should only be taken under the supervision of a health care practitioner.
Place of origin
Unknown
Side effects
Side-effects associated with prednisone include irregular heartbeat, increased blood pressure, stomach ulcer, blood disorders, skin, muscle and bone damage, and nervous system disorders. Consult with your health care practitioner prior to stopping use, as sudden discontinuation of prednisone may cause symptoms of withdrawal.
Side-effects associated with diclofenac and ibuprofen include changes in blood pressure, gastrointestinal disorders (with or without bleeding), anemia, kidney failure and reduced blood clotting ability.
Side-effects associated with hydrochlorothiazide include changes in electrolytes (specifically potassium), muscle cramps, dizziness, low blood pressure, headache and nausea.
Side-effects associated with metoclopramide include drowsiness, fatigue, insomnia, headache and dizziness. Metoclopramide has been associated with a serious condition involving abnormal muscle movements.
Side-effects associated with trimethoprim include nausea, vomiting and skin rash.
Fu Fang Feng Shi Gu Kang Ling Jiao Nang
Product description
This product is promoted for joint pain.
Hazard identified
The Hong Kong Department of Health advised consumers not to use this product after it was found to contain undeclared prednisone, diclofenac, ibuprofen, indomethacin, piroxicam and metoclopramide.
- Prednisone is a steroidal prescription drug used to treat inflammatory conditions such as arthritis and allergic reactions.
- Diclofenac, indomethacin, ibuprofen, and piroxicam are drugs in the family known as non-steroidal anti-inflammatory drugs (NSAIDs) that are used to treat pain, fever and inflammation. All are prescription drugs except ibuprofen, which is a non-prescription NSAID.
- Metoclopramide is a prescription drug used to aid stomach emptying.
Prescription drugs should only be taken under the supervision of a health care practitioner.
Place of origin
Unknown
Side effects
Side-effects associated with prednisone include irregular heartbeat, increased blood pressure, stomach ulcer, blood disorders, skin, muscle and bone damage, and nervous system disorders. Consult with your health care practitioner prior to stopping use, as sudden discontinuation of prednisone may cause symptoms of withdrawal.
Side-effects associated with diclofenac and ibuprofen include changes in blood pressure, gastrointestinal disorders (with or without bleeding), anemia, kidney failure and reduced blood clotting ability.
Side-effects associated with hydrochlorothiazide include changes in electrolytes (specifically potassium), muscle cramps, dizziness, low blood pressure, headache and nausea.
Side-effects associated with metoclopramide include drowsiness, fatigue, insomnia, headache and dizziness. Metoclopramide has been associated with a serious condition involving abnormal muscle movements.
What you should do
Health Canada advises Canadians to contact the Health Products and Food Branch Inspectorate at 1-800-267-9675 if they find the product listed above in the Canadian marketplace.
Canadians who have this product are advised not to use it, and should consult with a health care practitioner if they have concerns about their health related to the use of this product.
Background
Drugs and natural health products that are authorized for sale in Canada will have an eight-digit Drug Identification Number (DIN), a Natural Product Number (NPN) or a Homeopathic Medicine Number (DIN-HM) on the label. These numbers indicate that the products have been assessed by Health Canada for safety, effectiveness and quality. Some products may also still have an Exemption Number (EN). Health Canada has allowed a transition period for industry to move natural health products through the market that were labelled with temporary EN numbers.
As of July 30, 2013, no adverse reactions suspected to be associated with the use of these products have been reported to Health Canada.
Report a health or safety concern
- Call toll-free at 1-866-234-2345
- Visit Health Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax
Media enquiries
Health Canada
(613) 957-2983
Public enquiries
(613) 957-2991
1-866 225-0709
Images
Select thumbnail to enlarge - opens in a new window