This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Foreign Product Alert: Enhanced Vegetal Vigra capsules, Extreme Diamond 3000, Gold Viagra capsules, Gold Viagra tablets (packaged as "Kangaroo Sexually Invigorating Essence"), Majestic Lovezone tablets, Niu Mo Wang 'Bull Monster' tablets, North West ...
- Starting date:
- July 2, 2015
- Posting date:
- July 2, 2015
- Type of communication:
- Foreign Product Alert (FPA)
- Subcategory:
- Drugs, Natural health products
- Source of recall:
- Health Canada
- Issue:
- Unauthorized products, Undeclared Substance, Important Safety Information
- Audience:
- General Public
- Identification number:
- RA-53996
Issue
These foreign health products have been found by regulators in other countries to contain undeclared drug ingredients.
The products are not authorized for sale in Canada and have not been found in the Canadian marketplace but it is possible they may have been brought into the country by travellers or purchased over the Internet.
| Product Name(s) | Hazard(s) Identified | Source of Alert | Other product information, if available | Images |
|---|---|---|---|---|
| Unauthorized Sexual Enhancement Products | ||||
| Enhanced Vegetal Vigra capsules | Undeclared sildenafil | Australian Therapeutic Goods Administration | Capsules | |
| Extreme Diamond 3000 | Undeclared desmethyl carbodenafil and dapoxetine | United States Food and Drug Administration | ||
| Gold Viagra capsules | Undeclared sildenafil | Australian Therapeutic Goods Administration | Capsules | |
| Gold Viagra tablets (packaged as "Kangaroo Sexually Invigorating Essence") | Undeclared sildenafil and tadalafil | Australian Therapeutic Goods Administration | Crescent-shaped, yellow tablets with 'Goldviagra' debossed on either side | |
| Majestic Lovezone tablets | Undeclared sildenafil | Australian Therapeutic Goods Administration | Tablets | |
| Niu Mo Wang 'Bull Monster' tablets | Undeclared sildenafil | Australian Therapeutic Goods Administration | Two-part product (foil-packaged tablets & small round black pills in clear bag) | |
| North West Wolf capsules | Undeclared sildenafil | Australian Therapeutic Goods Administration | Capsules | |
| Strong Horses capsules | Undeclared sildenafil | Australian Therapeutic Goods Administration | Capsules | |
| Strong-SX capsules | Undeclared sildenafil | Australian Therapeutic Goods Administration | Capsules | |
| USA Gold Ant capsules | Undeclared sildenafil | Australian Therapeutic Goods Administration | Capsules | |
| Unauthorized Weight Loss Products | ||||
| Black Mamba Hyperrush | Undeclared sibutramine and phenolphthalein | United States Food and Drug Administration | ||
| Botanical Slimming (Red) | Undeclared fluoxetine | United States Food and Drug Administration | ||
| Green Algae Combination by Crane Beauty | Undeclared lorcaserin | United States Food and Drug Administration | ||
| Natural Max Slimming | Undeclared sildenafil, fluoxetine, and sibutramine | United States Food and Drug Administration | Capsules | |
| OMG Slim capsules | Undeclared sibutramine and orlistat | Australian Therapeutic Goods Administration | Capsules | |
| Skinny 22" | Undeclared phenolphthalein | Singapore Health Sciences Authority | ||
| Ultra ZX | Undeclared sibutramine and phenolphthalein | United States Food and Drug Administration | ||
| Ultimate Boost | Undeclared phenolphthalein | United States Food and Drug Administration | Capsules | |
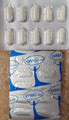
| Xcel | Undeclared fluoxetine | United States Food and Drug Administration | Capsules | |
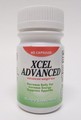
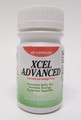
| Xcel Advanced | Undeclared phenolphthalein | United States Food and Drug Administration | Capsules |
What you should do
- Contact the Health Products and Food Branch Inspectorate at 1-800-267-9675 if you find a product listed above in the Canadian marketplace.
- Consult with a healthcare professional if you have health concerns related to the use of any of these products.
- Read the label of the products you buy to verify that they have been assessed by Health Canada for safety, effectiveness and quality. Health products that have been authorized for sale by Health Canada will have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or a Homeopathic Drug Number (DIN-HM).
Background
Prescription drugs should only be used under the supervision of a healthcare professional.
Dapoxetine is a prescription drug used to treat premature ejaculation and is not authorized for sale in Canada. Side effects include seizures, changes in blood pressure, blurred vision, dizziness, headache and nausea.
Desmethyl carbodenafil is an unauthorized substance similar to sildenafil and may pose similar health risks. Sildenafil is a prescription drug used to treat erectile dysfunction. Sildenafil should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Fluoxetine is a prescription drug used to treat depression. Side effects include headache, sleeping problems, anxiety, nausea, loss of appetite and excessive sweating.
Lorcaserin is a prescription weight-loss drug that is not authorised for sale in Canada. Side effects include fatigue, headache, cough, sore throat, breathing difficulties, nausea, abdominal discomfort, back pain, irregular heartbeat, leg swelling, anxiety, mood change, and depression. In diabetic patients, severe low blood sugar may occur.
Orlistat is a prescription weight-loss drug. Side effects include abdominal discomfort, anal leakage, increased stool volume and frequency, and fatty/oily stool.
Phenolphthalein was previously used as a laxative but is no longer authorized for sale in Canada because it may cause cancer. Additional side effects include decreased blood pressure, skin rash and gastrointestinal bleeding.
Sibutramine was previously used to treat obesity but is no longer authorized for sale in Canada because of its association with an increased risk of cardiovascular side effects such as heart attack and stroke. Other side effects include increased blood pressure and heart rate, dry mouth, difficulty sleeping and constipation.
Sildenafil is a prescription drug used to treat erectile dysfunction. Sildenafil should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Tadalafil is a prescription drug used to treat erectile dysfunction. Tadalafil should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side-effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other side-effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Report health or safety concerns
To report a side effect to a health product to Health Canada:
- Call toll-free at 1-866-234-2345
- Visit Health Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax
As of June 23, 2015, no adverse reactions suspected to be associated with the use of these products have been reported to Health Canada.
Images
Select thumbnail to enlarge - opens in a new window