Foreign Product Alert: Black Ant King tablets, Gold Viagra 9800mg capsules, LIPRO Dietary capsules, Stree Overlord Strong tablets (pills), Vegetal Vigra capsules, ViaGro 500mg Male Enhancement capsules
- Starting date:
- November 22, 2018
- Type of communication:
- Foreign Product Alert (FPA)
- Subcategory:
- Drugs
- Source of recall:
- Health Canada
- Issue:
- Undeclared Substance
- Audience:
- General Public
- Identification number:
- RA-68354
Issue
These foreign health products have been found by regulators in other countries to contain undeclared drug ingredients.
The products are not authorized for sale in Canada and have not been found in the Canadian marketplace, but it is possible they may have been brought into the country by travellers or purchased over the Internet.
| Product Name(s) | Hazard(s) Identified | Source of Alert | Images |
|---|---|---|---|
| Unauthorized Sexual Enhancement Products | |||
| Black Ant King tablets | Undeclared sildenafil and chloramphenicol | Australia Therapeutic Goods Administration | |
| Gold Viagra 9800mg capsules | Undeclared sildenafil | Australia Therapeutic Goods Administration | |
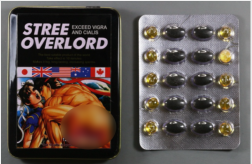
| Stree Overlord Strong tablets (pills) | Undeclared sildenafil | Australia Therapeutic Goods Administration | |
| Vegetal Vigra capsules | Undeclared sildenafil | Australia Therapeutic Goods Administration | |
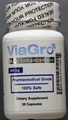
| ViaGro 500mg Male Enhancement capsules | Undeclared theophylline and caffeine | Australia Therapeutic Goods Administration | |
| Unauthorized Weight Loss Products | |||
| LIPRO Dietary capsules | Undeclared sibutramine | Australia Therapeutic Goods Administration |
What you should do
- Contact Health Canada at 1-800-267-9675 or by completing an online complaint form if you find a product listed above in the Canadian marketplace.
- Consult a healthcare professional if you have health concerns related to the use of any of these products.
- Read the label of the products you buy to verify that they have been assessed by Health Canada for safety, effectiveness and quality. Health products that have been authorized for sale by Health Canada will have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM).
Background
Prescription drugs should only be used under the supervision of a healthcare professional.
Caffeine, a natural health product, is used in health products to promote alertness and endurance, to enhance cognitive and motor performance, and to promote urine production.
Chloramphenicol is a prescription antibiotic drug.
Sibutramine was previously used to treat obesity but is no longer authorized for sale in Canada because of its association with an increased risk of cardiovascular side effects such as heart attack and stroke. Other side effects include increased blood pressure and heart rate, dry mouth, difficulty sleeping, and constipation.
Sildenafil is a prescription drug used to treat erectile dysfunction. Sildenafil should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure, and abnormal heartbeat. Other side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Theophylline is a prescription drug used to treat asthma and bronchitis.
Report health or safety concerns
To report a side effect to a health product to Health Canada:
- Call toll-free at 1-866-234-2345
- Visit Health Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax
As of February 12, 2018, no adverse reactions suspected to be associated with the use of these products have been reported to Health Canada.
Images
Select thumbnail to enlarge - opens in a new window