This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Foreign Product Alert: Africa Black Ant, Anyang Herbal Blue, Anyang Herbal Red, Arouse-Plus, Australia Kangaroo Essence, Bazook Bullet, Change Me Herbal Slimming, Placebo Tablets, Ultimate & Herbal Slim Weight Loss capsules
- Starting date:
- May 12, 2017
- Posting date:
- May 12, 2017
- Type of communication:
- Foreign Product Alert (FPA)
- Subcategory:
- Natural health products, Specialized Products
- Source of recall:
- Health Canada
- Issue:
- Undeclared Substance
- Audience:
- General Public
- Identification number:
- RA-63260
These foreign health products have been found by regulators in other countries to contain undeclared drug ingredients.
The products are not authorized for sale in Canada and have not been found in the Canadian marketplace, but it is possible they may have been brought into the country by travellers or purchased over the Internet.
| Product Name(s) | Hazard(s) Identified | Source of Alert |
|---|---|---|
| Unauthorized Sexual Enhancement Products | ||
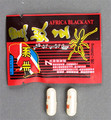
| Africa Black Ant capsules | Undeclared sildenafil | Australia Therapeutic Goods Administration |
| Arouse-Plus | Undeclared tadalafil | United States Food and Drug Administration |
| Australia Kangaroo Essence | Undeclared sildenafil | Australia Therapeutic Goods Administration |
| Bazook Bullet | Undeclared tadalafil | United States Food and Drug Administration |
| Unauthorized Weight Loss Products | ||
| Anyang Herbal Blue | Undeclared sibutramine | Singapore Health Sciences Authority |
| Anyang Herbal Red | Undeclared diclofenac, phenolphthalein, and sibutramine | Singapore Health Sciences Authority |
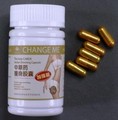
| Change Me Herbal Slimming Capsules | Undeclared sibutramine | Australia Therapeutic Goods Administration |
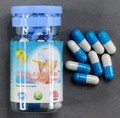
| Ultimate & Herbal Slim Weight Loss capsules | Undeclared sibutramine | Australia Therapeutic Goods Administration |
| Other Unauthorized Products | ||
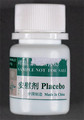
| Placebo tablets | Undeclared clenbuterol | Australia Therapeutic Goods Administration |
What you should do
- Contact Health Canada at 1-800-267-9675 or by completing an online complaint form if you find a product listed above in the Canadian marketplace.
- Consult a healthcare professional if you have health concerns related to the use of any of these products.
- Read the label of the products you buy to verify that they have been assessed by Health Canada for safety, effectiveness and quality. Health products that have been authorized for sale by Health Canada will have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM).
Background
Prescription drugs should only be used under the supervision of a healthcare professional.
Clenbuterol is a veterinary drug used to treat respiratory diseases in horses. It is not authorized for human use in Canada. Serious side effects include seizure, heart attack, psychosis, or damage to skeletal muscle. Other side effects include irregular heartbeat, increased blood pressure, tremors, nervousness, nausea, increased blood sugar, headache and dizziness.
Diclofenac is a prescription drug in the family known as non-steroidal anti-inflammatory drugs (NSAIDs) and is used to treat pain, fever and inflammation. Side-effects include changes in blood pressure, gastrointestinal disorders (with or without bleeding), anemia, kidney failure and reduced blood clotting ability.
Phenolphthalein was previously used as a laxative but is no longer authorized for sale in Canada because it may cause cancer. Additional side effects include decreased blood pressure, skin rash and gastrointestinal bleeding.
Sibutramine was previously used to treat obesity but is no longer authorized for sale in Canada because of its association with an increased risk of cardiovascular side effects, such as heart attack and stroke. Other side effects include increased blood pressure and heart rate, dry mouth, difficulty sleeping and constipation.
Sildenafil is a prescription drug used to treat erectile dysfunction. Sildenafil should not be used by individuals taking any kind of nitrate drug (such as nitroglycerine) because it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other side effects include headache, facial flushing, indigestion, dizziness, abnormal vision and hearing loss.
Tadalafil is a prescription drug used to treat erectile dysfunction. Tadalafil should not be used by individuals taking any kind of nitrate drug (such as nitroglycerine) because it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other side effects include headache, facial flushing, indigestion, dizziness, abnormal vision and hearing loss.
Report health or safety concerns
To report a side effect to a health product to Health Canada:
- Call toll-free at 1-866-234-2345
- Visit Health Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax
As of March 3, 2017, no adverse reactions suspected to be associated with the use of these products have been reported to Health Canada.
Images
Select thumbnail to enlarge - opens in a new window