Distribution of SPIKEVAX (elasomeran) COVID-19 Vaccine with English-only Vial and Carton Labels
Brand(s)
Summary
Affected products
Issue
IMPORTANT: Access to Canadian-specific labelling information during the distribution of the SPIKEVAX (elasomeran) COVID-19 Vaccine with English-only vial and carton labels.
Audience
Healthcare professionals including infectious disease physicians, pharmacists, family physicians, public health officials, nurses and nurse practitioners. Healthcare professionals at the identified points of use.
Key messages
- On December 23, 2020, COVID-19 Vaccine Moderna (DIN 02510014) was authorized in accordance with the Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19.
- On September 16, 2021 COVID-19 Vaccine Moderna was issued a Notice of Compliance (NOC) under the Food and Drug Regulations and is now authorized under the brand name SPIKEVAX (elasomeran).
- At this time, Moderna is providing vaccine supplies with global English-only vial and carton labels, with the brand name SPIKEVAX, in order to maintain access to the vaccine in Canada (see Appendix A and Appendix B).
- Existing vaccine supply labelled as COVID-19 Vaccine Moderna on the vial and carton, that is within expiry, may continue to be used.
- To avoid excessive puncturing and compromising product integrity, DO NOT puncture and withdraw doses from the COVID-19 Vaccine Moderna / SPIKEVAX vials more than 20 times.
- Healthcare professionals are advised that:
- Important Canadian-specific information is absent from the vial and carton labels (see the Information for healthcare professionals section).
- The Canadian-specific labelling information including the SPIKEVAX Product Monograph and training materials can be accessed at www.modernacovid19global.com/ca, or by scanning the QR code on the English-only vial and carton labels. This information is also available on the federal government’s covid-vaccine.canada.ca website. The Canadian SPIKEVAX Product Monograph in French and English is also available on Health Canada’s Drug Product Database.
- Moderna will develop Canadian-specific vial and carton labels with the SPIKEVAX brand name in French and English, and make them available on the www.modernacovid19global.com/ca website at a future date.
What is the issue?
SPIKEVAX (elasomeran) was issued a Notice of Compliance (NOC) under the Food and Drug Regulations, replacing the previous authorization under the Interim Order. As an extraordinary measure to provide access to vaccine supplies in the context of the global pandemic, at this time Moderna is providing vaccine supplies with vials and cartons labelled with the brand name SPIKEVAX. This label is presented in English-only and is missing some important Canadian-specific information normally found on Health Canada approved labels (see the Information for healthcare professionals section).
Products affected
SPIKEVAX (elasomeran) (100 mcg / 0.5mL), dispersion for intramuscular injection, multidose vial (5 mL).
DIN: 02510014
Manufacturer: ModernaTx, Inc.
Canadian Importer and Distributor: Innomar Strategies
Background information
SPIKEVAX (elasomeran mRNA vaccine) is indicated for active immunization against coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 12 years of age and older.
Given the public health emergency resulting from the current pandemic, Health Canada has authorized the importation, sale, and advertising of SPIKEVAX with vial and carton labels that are in English-only for the global distribution of the vaccine.
SPIKEVAX with English-only labels is the same as the Health Canada authorized COVID-19 Vaccine Moderna / SPIKEVAX in all aspects (i.e., formulation, strength, route of administration) and should be used in Canada for the same indication and per the same vaccination schedule. The Canadian SPIKEVAX Product Monograph, which is approved by Health Canada and available in French and English, should be used for complete product information.
Information for healthcare professionals
In order to provide rapid access to SPIKEVAX for Canadians, Moderna will provide product vials and cartons labelled in English-only for a limited time period (see Appendix A and Appendix B).
Healthcare professionals are advised that:
- The Canadian-specific information including the SPIKEVAX Product Monograph and training materials can be accessed at www.modernacovid19global.com/ca, or by scanning the QR code on carton and vial labels. This information is also available on the federal government’s covid-vaccine.canada.ca website. The Canadian SPIKEVAX Product Monograph in French and English is also available on Health Canada’s Drug Product Database.
- The following important Canadian-specific information is absent from the vial and carton labels:
- Drug Identification Number (DIN)
- name and address of the Canadian DIN holder
- name and address of the Canadian importer and distributor
- all corresponding text in French
- To avoid excessive puncturing and compromising product integrity, DO NOT puncture and withdraw doses from the COVID-19 Vaccine Moderna / SPIKEVAX vials more than 20 times.
Action taken by Health Canada
Health Canada is permitting the use of global English-only vial and carton labels for SPIKEVAX for a limited period. Health Canada has imposed terms and conditions requiring Moderna to provide vaccine supplies with Canadian-specific labels as soon as possible. Health Canada has made full labelling information available in French and English on the federal government’s covid-vaccine.canada.ca website.
Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication will be further distributed through the MedEffect™ e-Notice email notification system, as well as through social media channels, including LinkedIn and Twitter.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any serious or unexpected side effects in patients receiving SPIKEVAX should be reported to your local Health Unit or Moderna.
Moderna Biopharma Canada Corporation
c/o SE Corporate Services Ltd.,
Suite 1700, Park Place, 666 Burrard Street,
Vancouver, BC V6C 2X8
Telephone: 1-866-663-3762
Fax: 1-866-599-1342
To correct your mailing address or fax number, contact Moderna Biopharma Canada Corporation at 1-866-MODERNA (1-866-663-3762).
If a patient experiences a side effect following immunization, please complete the Adverse Events Following Immunization (AEFI) Form appropriate for your province/territory
(https://www.canada.ca/en/public-health/services/immunization/reporting-adverse-events-following-immunization/form.html) and send it to your local Health Unit.
For other health product inquiries related to this communication, contact Health Canada at:
Biologic and Radiopharmaceutical Drugs Directorate
E-mail: brdd.dgo.enquiries@hc-sc.gc.ca
Original signed by
Leslie Madden, BSc, MBA, LLM
Director, Head of Regulatory Affairs Canada
ModernaTx, Inc.
Appendix A – Vial and carton labels for SPIKEVAX (elasomeran) with English-only labelling, version 1
SPIKEVAX Vial Label

|
COVID-19 Vaccine Moderna Store frozen at -25°C to -15°C. Read the package leaflet before use. Moderna Biotech Spain SL [QR code] Scan for package leaflet or visit www.ModernaCovid19Global.com |
spikevax™ 0.20 mg/mL Dispersion for Injection COVID-19 mRNA Vaccine Discard Date/Time: Multidose vial (10 doses of 0.5 mL)
LOT EXP |
SPIKEVAX Carton Label
|
Moderna spikevax™ 0.20 mg/mL Dispersion for Injection COVID-19 mRNA Vaccine (nucleoside modified) |
COVID-19 Vaccine Moderna 10 multidose vials (each vial contains 10 doses of 0.5 mL) |
|
Black box contains: PC EXP LOT
Moderna™ Moderna Biotech Spain SL Calle Monte Esquinza 30, Bajo Izquierda 28010, Madrid, Spain |
Each multidose vial contains 10 doses (0.5 mL each). Also contains Lipid SM-102, cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-Dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (PEG2000-DMG), trometamol, trometamol hydrochloride, acetic acid, sodium acetate trihydrate, sucrose, water for injections. Store frozen at -25°C to -15°C. Read the package leaflet for the shelf life after first opening and for additional storage information. Keep out of sight and reach of children. [QR code] Read the package leaflet before use. Scan here for package leaflet or visit www.ModernaCOVID-19Global.com |
Appendix B – Vial and carton labels for SPIKEVAX (elasomeran) with English-only labelling, version 2
SPIKEVAX Vial Label
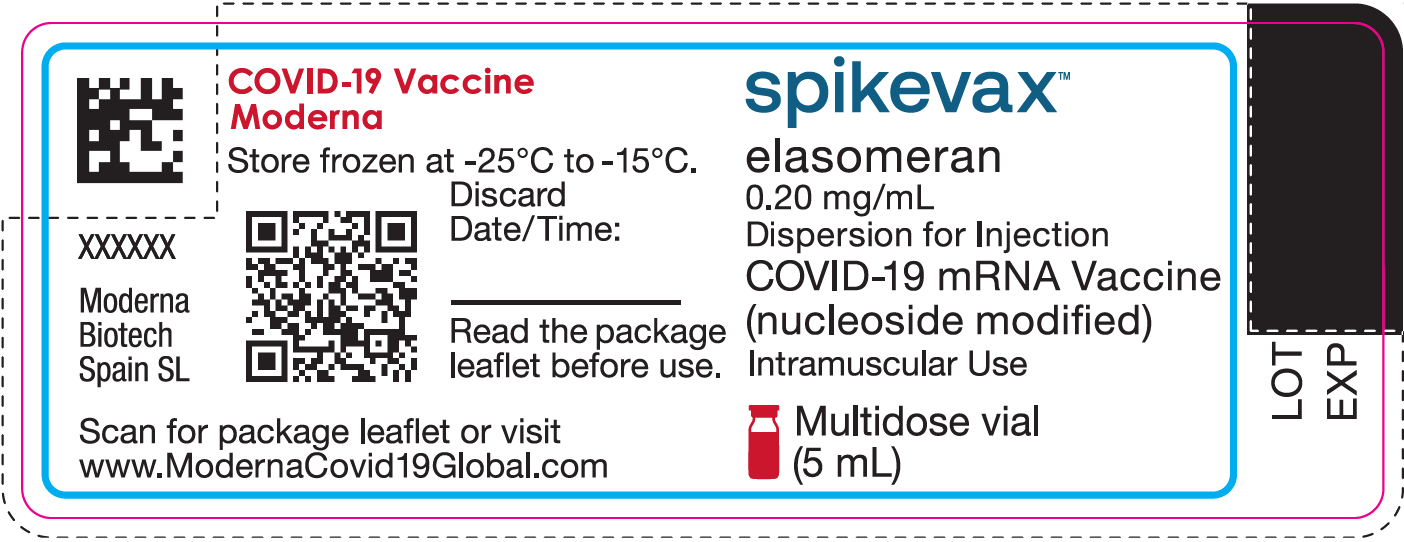
|
COVID-19 Vaccine Moderna Store frozen at -25°C to -15°C. Discard Date/Time: ________________ Read the package leaflet before use. Moderna Biotech Spain SL [QR code] Scan for package leaflet or visit www.ModernaCovid19Global.com |
spikevax™ elasomeran 0.20 mg/mL Dispersion for Injection COVID-19 mRNA Vaccine Multidose vial (5 mL)
LOT EXP |
SPIKEVAX Carton Label

|
Moderna spikevax™ elasomeran 0.20 mg/mL Dispersion for Injection COVID-19 mRNA Vaccine (nucleoside modified)
|
COVID-19 Vaccine Moderna 10 multidose vials (each vial contains 5 mL) |
|
Black box contains: PC EXP LOT
Moderna™ Moderna Biotech Spain SL Calle Monte Esquinza 30, Bajo Izquierda 28010, Madrid, Spain |
Each multidose vial contains 5 mL. Store frozen at -25°C to -15°C. Read the package leaflet for the shelf life after first opening and for additional storage information. Keep out of sight and reach of children. Dispose of in accordance with local requirements. [QR code] Read the package leaflet before use. Scan here for package leaflet or visit www.ModernaCOVID-19Global.com |
Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.
