Counterfeit COVID-19 antigen rapid test kits found in Ontario
Summary
If you suspect you have a counterfeit kit, do not use it and dispose of it in household garbage. Report suspected counterfeit medical devices to Health Canada. You can also report suspected counterfeit BTNX test kits to BTNX Inc. by calling toll-free at 1-888-339-9964, or by email at covid19@btnx.com with the subject line “Suspected Counterfeit BTNX Tests.”
Affected products
Counterfeit BTNX Rapid Response COVID-19 antigen rapid test kits (25-pack). This table compares the counterfeit vs. authentic BTNX Inc. kits and gives tips for what to look for.
| Image Counterfeit BTNX test kit (25-pack) | Counterfeit BTNX test kit (25-pack) | Image Authentic BTNX test kit (25-pack) | Authentic BTNX test kit (25-pack) |
|---|---|---|---|
 |
Counterfeit box (top) Health Advance name and phone number along with text "Official Canadian Distributor" and unauthorized text "Health Canada Approved" appear on box. |
 |
Authentic box (top) Authentic boxes are sealed with a clear sticker with blue lettering that says "QC APPROVED". |
 |
Counterfeit box (side) Health Advance name and phone number appear on box. |
 |
Authentic box (side) BTNX Inc. name, address and symbol appear on box. |
 |
Counterfeit kits - three open boxes Each box has varying contents, including varying test cassettes and assay buffers. |
 |
Authentic kit - open box Shown here: test cassettes, tube stand, assay buffer, swabs, procedure card and package insert. Note, assay buffer may come in bottles (shown here) or 25 single-use vials (not shown here). Not shown here: extraction tubes and nozzle filters |
 |
Counterfeit kits - cassette pouch found in some kits Expiry date and lot number may not match the box. Pouches are not green. |
 |
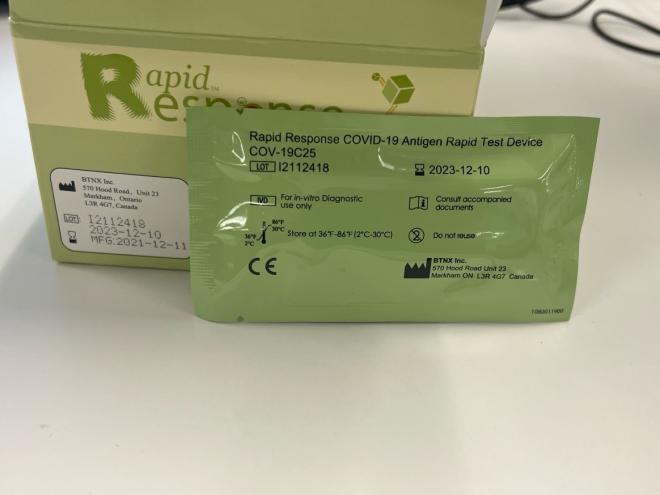
Authentic kit - box and cassette pouch (back) Lot number and expiry date on cassette pouch should match the lot number and expiry date on the box. Lot numbers start with the letter "I". |
Issue
Health Canada is warning consumers about counterfeit BTNX Rapid Response COVID-19 antigen rapid test kits (25-pack boxes) found in Ontario. The counterfeit devices were sold online by a distributor named Healthful Plus, without the required licence to import, distribute or sell medical devices in Canada.
The packaging of the counterfeit kits resembles authentic (licensed) BTNX Inc. products in colour and typeface and uses the BTNX Inc. device identifier "COV-19C25"; however, unlike authentic BTNX Inc. products, the counterfeit kits:
- are labelled as manufactured by "Health Advance Inc." instead of BTNX Inc.;
- lists Health Advance as an "Official Canadian Distributor"; and
- includes the text "Health Canada Approved". Claims of endorsement by government authorities, such as Health Canada, are not permitted.
Counterfeit health products are imitations of authentic products. The safety and effectiveness of these counterfeit kits have not been assessed by Health Canada.
After becoming aware of the potential counterfeit kits, Health Canada confirmed with BTNX Inc. that the devices were counterfeit. Health Canada also received confirmation from the purchaser of the counterfeit products that they had purchased the kits for personal use. The entire shipment, which contained 435 boxes of the 25-pack, was sent to Health Canada for compliance follow-up.
Based on information to date, the issue appears to be limited to one manufacturer, Health Advance Inc., and one distributor, Healthful Plus. Health Advance Inc. appears to no longer be manufacturing medical devices and Healthful Plus's website has been removed and the company appears to no longer be in operation.
COVID-19 rapid antigen tests are an essential tool in helping to detect infection and slow the spread of the disease. Health Canada recognizes that it is vital that Canadians can trust that the test kits that they rely on are authentic. Heath Canada is informing the provinces and territories about this issue, as well as Medical Device Establishment License holders, advising them to not purchase from the two companies or further distribute.
Health Canada is committed to protecting Canada's supply of licensed COVID-19 test kits and preventing counterfeit products from entering the Canadian supply chain. It will continue to assess any potential counterfeit products it becomes aware of and to take compliance and enforcement action as needed, including informing consumers.
What you should do
- Canadians are advised not to use any of the components contained in these counterfeit kits regardless of how much they may resemble components found in other licensed COVID-19 test kits sold in Canada.
- Health Canada has no evidence to suggest that additional counterfeit kits have been distributed in Canada.
- If you suspect you have a counterfeit kit, do not use it and dispose of it in household garbage. Report suspected counterfeit medical devices to Health Canada. You can also report suspected counterfeit BTNX test kits to BTNX Inc. by calling toll-free at 1-888-339-9964, or by email at covid19@btnx.com with the subject line "Suspected Counterfeit BTNX Tests."
What companies should know
- It is illegal to sell or advertise counterfeit health products
- When non-compliance is confirmed by Health Canada, a number of compliance and enforcement options are available to correct non-compliance and mitigate the risk to Canadians including, for example, on-site visits, recalls, public communications, and product seizures. The primary objective of Health Canada's compliance and enforcement approach is to manage the risks to Canadians using the most appropriate level of intervention.
Additional information
Details
Media and public enquiries
Media Enquiries
Health Canada
613-957-2983
media@hc-sc.gc.ca
Public Enquiries
613-957-2991
1-866-225-0709
Get notified
Receive notifications for new and updated recalls and alerts by category.