Certain bottles of JAMP Venlafaxine XR 37.5 mg capsules recalled due to mislabelling that could lead to overdose
Summary
- Check the capsules in your pharmacy bottle of JAMP Venlafaxine XR 37.5 mg capsules to make sure it contains 37.5 mg capsules (grey / pink in colour) and not 150 mg capsules (caramel in colour). If it contains 150 mg capsules, or if you are unsure, return it to your pharmacy as soon as possible. Your pharmacist will check it and provide a replacement with the correct capsules, if needed.
- Contact your health care professional if you have taken the wrong dose of your medication. Seek medical attention immediately if you experience any serious side effects from Venlafaxine XR, which may include allergic reactions, gastrointestinal bleeding, seizures, heart rhythm problems, blurred vision, eye pain, or severe headache.
Affected products
| Product | DIN | Lot | Expiry date |
|---|---|---|---|
| JAMP Venlafaxine XR 37.5 mg | 02516535 | PTC5140A | 09/2024 |
Issue
JAMP Pharma Corporation is recalling mislabelled bottles from lot PTC5140A of Venlafaxine extended release (XR) capsules after one bottle labelled to contain 37.5 mg capsules was found to contain 150 mg capsules. Only the mislabelled bottles are being recalled.
Venlafaxine XR is a prescription drug used to relieve symptoms of major depressive disorder and anxiety caused by generalized anxiety disorder, social anxiety disorder and panic disorder.
If pharmacists did not recognize the error, they may have repackaged and dispensed pharmacy bottles labelled as containing 37.5 mg capsules, but instead contained 150 mg capsules. This error could lead to patients taking a higher dose than prescribed.
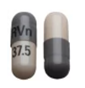
Venlafaxine XR 37.5 mg capsules have a grey cap and pink body, with "RVn" printed on the cap and "37.5" printed on the body.
Venlafaxine XR 150 mg capsules are caramel in colour with "RVn" printed on the cap and "150" printed on the body.
| Venlafaxine XR de JAMP (capsules de 37,5 mg) | Venlafaxine XR de JAMP (capsules de 150 mg) |
|---|---|
 |
 |
Accidently taking more than the prescribed dose can lead to serious side effects. Side effects requiring immediate medical attention include allergic reactions, gastrointestinal bleeding (signs include vomiting blood, passing blood in stool or black stool), seizures, heart rhythm problems, blurred vision, eye pain and severe headache. A sudden dose increase may also cause chills, high blood pressure, decreased appetite, nausea, agitation, dizziness, sleepiness, tremor, yawning or sweating.
The Department is monitoring the company's recall and will inform the public if any new health risks are identified.
What you should do
- Check the capsules in your pharmacy bottle of JAMP Venlafaxine XR 37.5 mg capsules to make sure it contains 37.5 mg capsules (grey / pink in colour) and not 150 mg capsules (caramel in colour). If you are unsure, contact your pharmacist to check if your bottle contains the correct capsules.
- If you see incorrect capsules, stop taking the medication and contact your pharmacy immediately for a replacement product. Return the affected product to your pharmacy for proper disposal.
- Contact your health care professional if you have taken the wrong dose of your medication. Seek medical attention immediately if you experience any serious side effects from Venlafaxine XR, which may include allergic reactions, gastrointestinal bleeding, seizures, heart rhythm problems, blurred vision, eye pain, or severe headache.
- Contact JAMP Pharma Corporation by email at alewicki@jamppharma.com if you have questions about this recall.
- Report any health product-related side effects or complaints to Health Canada.
Additional information
Details
Media and public enquiries
Media Enquiries:
Health Canada
613-957-2983
media@hc-sc.gc.ca
Public Enquiries:
613-957-2991
1-866-225-0709
info@hc-sc.gc.ca
Get notified
Receive emails about new and updated recall and safety alerts.