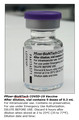
Authorization of Pfizer-BioNTech COVID-19 Vaccine with English-only Carton and Vial Labels
- Starting date:
- December 12, 2020
- Posting date:
- December 12, 2020
- Type of communication:
- Dear Healthcare Professional Letter
- Subcategory:
- Biologic/vaccine
- Source of recall:
- Health Canada
- Issue:
- Supply, Product Safety, Important Safety Information
- Audience:
- Healthcare Professionals
- Identification number:
- RA-74541
Last updated:
2020-12-18
Audiences
Healthcare professionals including infectious disease physicians, pharmacists, family physicians, public health officials, nurses and nurse practitioners. Healthcare professionals at the identified Points of Use (POUs).
Pfizer is initially distributing Pfizer-BioNTech COVID-19 Vaccine doses directly to POUs, vaccination locations where administration of the vaccine will occur, as outlined by the provincial governments and public health authorities.
Key messages
- On December 9, 2020, Pfizer-BioNTech COVID-19 Vaccine (COVID-19 mRNA Vaccine) (DIN 02509210) was authorized in accordance with the Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19.
- Pfizer-BioNTech COVID-19 Vaccine is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) in individuals 16 years of age and older.
- At this time, Pfizer/BioNTech is providing vaccine supplies with English-only labels on the cartons and vials in order to expedite the global distribution of Pfizer-BioNTech COVID-19 Vaccine.
- Health Canada has imposed terms and conditions on the authorization of the vaccine requiring that Pfizer/BioNTech develop Canadian-specific labelling in French and English for the vaccine cartons and vials. Pfizer agreed that vaccine supplies with Canadian-specific labelling in French and English will be available as soon as feasible given the need to ensure a rapid global supply of the vaccine.
-
Healthcare professionals are advised that:
- Important Canadian-specific information is absent from the vial and carton labels (see the Information for healthcare professionals section).
- The Canadian Product Monograph, which is available in French and English on Health Canada's Drug Product Database, the government's covid-vaccine.canada.ca website, or at pfizer.ca and CVDvaccine.ca, should be referenced for complete product information.
- The Canadian-specific labelling information including the Product Monograph and training materials can be accessed at CVDvaccine.ca, or by scanning the QR code on the English-only carton label. This information is also available on the Health Canada website at covid-vaccine.canada.ca.
- Pfizer/BioNTech will develop Health Canada approved vial and carton labels in French and English, and make them available on the CVDvaccine.ca website in the coming weeks.
- The vial and/or carton labels include the statements "For use under Emergency Use Authorization." The US FDA specific information (e.g., Rx only, NDC) should be disregarded as this is not relevant to the Canadian authorization.
- Paper copies of the Canadian Product Monograph and Patient Handout in French and English will be available at the points of use for healthcare professionals and patients.
- Paper copies of the Health Canada approved vial and carton labels in French and English will also be made available once finalized in the coming weeks, for reference by healthcare professionals at the points of use.
Issue
Pfizer-BioNTech COVID-19 Vaccine was authorized for use in accordance with the Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19. As an extraordinary measure to provide earlier access to vaccine supplies in the context of the global pandemic, Pfizer/BioNTech is providing, at this time, vaccine cartons and vials labelled with the US Emergency Use label. This label is presented in English-only and is missing some important Canadian-specific information normally found on Health Canada approved labels.
Products affected
Pfizer-BioNTech COVID-19 Vaccine, suspension for intramuscular injection, multiple dose vials. After dilution, the vial contains 5 doses (each dose is 0.3 mL).
DIN: 02509210
Manufacturer: BioNTech Manufacturing GmbH (Germany)
Canadian Importer and Distributor: Pfizer Canada ULC
Background information
Pfizer-BioNTech COVID-19 Vaccine is indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older.
Given the public health emergency resulting from the current pandemic, Health Canada has authorized the importation, sale, and advertising of Pfizer-BioNTech COVID-19 Vaccine with carton and vial labels that are in English-only and meant for the initial global distribution of the vaccine. This allows earlier access to the vaccine for the Canadian population ahead of the Canadian-labelled Pfizer-BioNTech COVID-19 Vaccine being available, and facilitates the global deployment of this vaccine across many countries given the high demand.
Pfizer-BioNTech COVID-19 Vaccine with global labels is the same as the Health Canada authorized Pfizer-BioNTech COVID-19 Vaccine in all aspects (i.e., formulation, strength, route of administration) and should be used in Canada for the same indication and per the same vaccination schedule. The Canadian Product Monograph for Pfizer-BioNTech COVID-19 Vaccine, which is approved by Health Canada and available in French and English, should be used for complete product information. The Product Monograph is available on Health Canada's Drug Product Database, on the government's covid-vaccine.canada.ca website, or at pfizer.ca and CVDvaccine.ca.
The use of Pfizer-BioNTech COVID-19 Vaccine is permitted under an interim authorization in accordance with the Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19.
Patients should be advised of the nature of the authorization.
Who is affected
Information for healthcare professionals
In order to provide rapid access to Pfizer-BioNTech COVID-19 Vaccine for Canadians, Pfizer Canada ULC will distribute product cartons and vials labelled in English-only for a limited time period.
Healthcare professionals are advised that:
- The approved Canadian Product Monograph, which is available in French and English on Health Canada's Drug Product Database, the government's covid-vaccine.canada.ca website or at pfizer.ca and CVDvaccine.ca, should be used for complete product information.
-
The following important Canadian-specific information is absent from the vial and carton labels:
- Drug Identification Number (DIN)
- name and address of the Canadian importer and distributor
- all corresponding text in French
- The Canadian-specific information can be accessed at CVDvaccine.ca, or by scanning the QR code on carton labels. This information is also available on the government's covid-vaccine.canada.ca website.
- Paper copies of the Canadian Product Monograph and Patient Handout will be available at the points of use for this vaccine.
- Pfizer/BioNTech will develop Health Canada approved vial and carton labels in French and English, and make them available on the CVDvaccine.ca website in the coming weeks for reference by healthcare professionals. Once finalized, a paper copy of these labels will also be made available for reference at the points of use.
- The vial and/or carton labels for the initial supplies of vaccine include the statements "For use under Emergency Use Authorization." The US FDA specific information (e.g., Rx only, NDC) should be disregarded as this is not relevant to the Canadian authorization.
- For any medical questions, contact Pfizer Canada ULC Medical Information at 1-800-463-6001.
- For any other general inquiries, contact Pfizer Canada ULC Customer Service at 1-833-VAX-COVI (1-833-829-2684) or email at CanadaCSVaccine@pfizer.com
Action taken by Health Canada
On September 16, 2020, Canada's Minister of Health approved an Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19 to expedite the authorization for the importation, sale, and advertising of drugs used in relation to COVID-19 while taking into consideration urgent public health needs. The Interim Order will expire after one year. Health Canada authorized the use of Pfizer-BioNTech COVID-19 Vaccine under the Interim Order on December 9, 2020, and this vaccine has been added to the "List of authorized drugs, vaccines and expanded indications" for COVID-19.
Health Canada is permitting the use of an English-only label for a limited period that reflects the US label for emergency use. Health Canada has imposed terms and conditions requiring Pfizer Canada ULC to provide vaccine supplies with Canadian-specific labels as soon as possible. Health Canada has made full labelling information available in French and English on the government's covid-vaccine.canada.ca website.
Health Canada has worked with Pfizer Canada ULC to prepare this alert for the Pfizer-BioNTech COVID-19 Vaccine. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication update will be further distributed through the MedEffect⢠e-Notice email notification system, as well as through social media channels, including LinkedIn and Twitter.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any serious or unexpected side effects in patients receiving Pfizer-BioNTech COVID-19 Vaccine should be reported to Pfizer Canada ULC or your local Health Unit.
Pfizer Canada ULC
17300 Trans-Canada Highway
Kirkland, QC
H9J 2M5
www.pfizersafetyreporting.com
Telephone: 1-866-723-7111
Fax: 1-855-242-5652
To correct your mailing address or fax number, contact Pfizer Canada ULC Customer Service at 1-833-VAX-COVI (1-833-829-2684).
If a patient experiences a side effect following immunization, please complete the Adverse Events Following Immunization (AEFI) Form appropriate for your province/territory (https://www.canada.ca/en/public-health/services/immunization/reporting-adverse-events-following-immunization/form.html) and send it to your local Health Unit.
For other health product inquiries related to this communication, contact Health Canada at:
Biologic and Radiopharmaceutical Drugs Directorate
E-mail: hc.brdd.dgo.enquiries.sc@canada.ca
Original signed by
Vratislav Hadrava M.D., Ph.D.
Vice President & Medical Director
Pfizer Canada ULC
Images
Select thumbnail to enlarge - opens in a new window